Journal of
eISSN: 2373-633X


Research Article Volume 9 Issue 3
1Health Sciences Centre, Federal University of Espirito Santo, Brazil
2Postgraduate Student of Nutrition and Health, Federal University of Espirito Santo, Brazil
3University Hospital Cassiano Antonio Moraes, Brazil
Correspondence: Valdete Regina Guandalini, Federal University of Espirito Santo, Avenue. Marechal Campos, 1468 Maruipe, 29040-090/ Vitoria, ES, Brazil,, Tel 55-279-9777-8404
Received: June 10, 2018 | Published: June 25, 2018
Citation: Barreto MA, Echebarrie AD, Carvalho LR, et al. Association of transthyretin and nutritional status in patients with cancer. J Cancer Prev Curr Res. 2018;9(3):152-157. DOI: 10.15406/jcpcr.2018.09.00340
Background: The malnutrition is generally seen in the hospital environment and it is a serious potentially condition in cancer patients. Measurement of serum proteins can be a useful indicator in the evaluation of nutritional status.
Objective: To evaluate the association of transthyretin with nutritional status in cancer patients.
Methods: This is a cross-sectional study, conducted with patients with a cancer confirmed diagnosis. Nutritional status was defined by the Subjective Global Assessment, anthropometric measurements, serum albumin and transthyretin.
Results: Seventy patients were evaluated, the median age was 58.51±14.85years, 51.4%(n=36) were elderly and female. Cancer of the lower gastrointestinal tract was the most prevalent, affecting 42.9%(n=30) patients. Subjective Global Assessment, transthyretin and serum albumin showed that 71.56%(n= 50), 50.0%(n= 35) and 32.9%(n=23) patients were malnourished, respectively. There were no differences between them. Significant correlations were found between transthyretin, arm circumference (p=0.025), arm muscle circumference (p=0.008) and corrected arm muscle area (p=0.001). Transthyretin showed sensitivity of 58.0%, specificity of 70.0%, positive predictive value of 83.0%, and negative predictive value of 61.0%.
Discussion: The findings showed the association of transthyretin with the anthropometric variables used to evaluate nutritional status, especially those that evaluate the reduction of energy and protein reserves.
Conclusion: Transthyretin was moderately associated with anthropometric variables. Given the limitations, transthyretin may be a complementary tool in nutritional evaluation, because it is feasible, easy to interpret and undergoes changes in a short period.
Keywords: nutritional assessment, subjective global assessment, malnutrition, transthyretin, cancer
AC, arm circumference; ACM, arm muscle circumference; BMI, body mass index; CAMA, corrected arm muscle area; CC, calf circumference (CC), CRP, C-reactive protein; NRS-2002, Nutrition Risk Screening; TAPM, thickness of the adductor pollicis muscle; TSF, triceps skinfold; SGA, Subjective Global Assessment
The magnitude of cancer is accompanied by the high prevalence of malnutrition, which accounts for 40–80% of patients with neoplasm.1,2 The nutritional status of the cancer patient is closely related to the quality of life and the prognosis of the disease,3 since malnutrition is associated with lower response and tolerance to antineoplastic treatment, infectious complications, increased hospital stay time and mortality.4,5
The nutritional evaluation of the oncological patient becomes essential to identifying the nutritional risk and to provide an early and adequate intervention.6 In clinical practice, the use of recognized instruments, such as the Subjective Global Assessment (SGA) and Nutrition Risk Screening (NRS-2002) are recommended, however they are subjective methods of nutritional assessment and may limit intervention reflexes and nutritional changes as they depend on the evaluator’s accuracy and experience.6,7
Complementary methods, such as the measurement of serum proteins, have been used to identify malnutrition quickly and efficiently, since they provide indirect information on visceral protein levels such as albumin and transthyretin, considered important tools for assessing nutritional status.8
Transthyretin has a half-life of 2 to 3days, and its serum levels decrease in response to liver disease, hydration status, hyperglycemia, acute and chronic inflammation and inadequate caloric-protein intake.9,10 Transthyretin is able to better identify patients at nutritional risk due to reduced food consumption rather than those already malnourished.9 Under conditions in which malnutrition may develop in a short time, transthyretin has become a preferred biomarker for assessing nutritional status when compared to the traditionally used albumin marker, which has a half-life of 21days.10
Among the major proteins synthesized in the liver, transthyretin is a negative acute phase serum protein, undergoing changes in response to intense stress.11 A low transthyretin concentration may therefore be considered first as a sign of nutritional risk, which requires prior monitoring and care of the patient.12
Given these characteristics, transthyretin has been able to detect nutritional change and determine treatment efficacy and nutritional prognosis,11,13 making it an important indicator to screen and diagnose malnutrition, since it is relatively low cost, can be used in the laboratory and can be used in various clinical conditions.9,14 This work was based on the hypothesis that the transthyretin can to predict malnutrition in cancer patients when compared to the classical methods of nutritional assessment. The objective of this study was to evaluate the association of transthyretin with nutritional status in cancer patients.
Study design
This is a cross-sectional descriptive study, using convenience sampling, performed at a General and Reparatory Surgery and Medical Clinic Unit, between July 2014 and April 2016, at a university hospital in Greater Vitória-ES/Brazil. Participants of the study were Adult (20 to 59.9years) and elderly (≥60years) patients with a confirmed clinical diagnosis of cancer, who presented serum protein evaluation in medical records and were submitted to nutritional status evaluation in the first 48hours of hospital admission. The exclusion criteria considered in this study were: being nursed in isolation and having a diagnosis of hepatic and renal cancer, in order to minimize the influences in the analysis of serum proteins.
After those patients who met the criteria for inclusion had signed an informed consent form, clinical, biochemical and sociodemographic data were collected from the information available in the medical records, followed by anthropometric evaluation at the bedside and administration of the SGA. The diagnosis of malnutrition obtained from transthyretin was compared with the results obtained from the SGA, anthropometric parameters and nutritional markers.
Nutritional assessment
The anthropometric evaluation was performed by previously trained evaluators, and consisted of body weight(Kg), height(m), arm circumference(AC) and calf circumference(CC) in centimeters, triceps skinfold (TSF) in millimeters, thickness of the adductor pollicis muscle (TAPM) and body mass index (BMI) (kg/m2). The BMI was calculated from the formula: current weight (kg)/height 2(m). All measurements were performed as recommended by Lohman et al.15
The weight was measured by a portable digital scale Techine® Mod. BAL-180 BR with 100gr of graduation and maximum of 180kg. The height was measured by means of a Personal Caprice Sanny® Estadiometer with a maximum height of 210cm. The circumference measurements were performed with a tape measure of non-elastic material and length of up to 150cm. For TSF and TAPM measurements, a Lange Scientific Adipometer® with a precision and sensitivity of 0.1mm and reading width of 85mm was used.
Subsequently, the arm muscle circumference (ACM) in centimeters and corrected arm muscle area (CAMA) in square centimeters, were determined. For the classification of ACM and CAMA, the percentile values proposed by Frisancho16 were used to evaluate the adequacy percentage. For the TAPM, were used. The reference values proposed by Bragagnolo et al.17
SGA was used as the gold standard for the diagnosis of nutritional status. Studies in cancer patients have used this method.18,19 The SGA includes aspects of the clinical history, such as weight changes, changes in food intake, presence of gastrointestinal symptoms, changes in functional capacity, physical examination, loss of subcutaneous fat and muscle mass, presence of sacral and ankle edema and ascites. The results are expressed in three categories: well-nourished patients (SGA “A”), suspected/moderate malnutrition (SGA “B”) or severely malnourished (SGA “C”). 20
Biochemical evaluation
For the biochemical evaluation, the serum concentrations of C-reactive protein (CRP), albumin and transthyretin, which were available in the medical record, were analyzed. All the tests were performed by the laboratory of the hospital where the research was carried out. The classification of nutritional status from transthyretin was performed in this study based on the reference values proposed by Shenkin.21 Thus, patients with transthyretin> 17mg/dL, with moderate risk of malnutrition between ≤17 and ≥10mg/dL and severe risk of malnutrition, were classified as well-nourished patients with transthyretin level>10mg/dL.
Statistical analysis
For data analysis, serum transthyretin concentrations were grouped into two categories: well-nourished (transthyretin> 17mg/dL) and malnourished (transthyretin ≤17mg/dL). For serum albumin levels, nutritional status was classified into well-nourished patients (albumin ≥3.5mg/dL) and malnourished patients (albumin ≤3.5mg/dL). Means and standard deviations were used to describe the continuous and percentage variables for the categorical variables. The Kolmogorov–Smirnov test was used to verify the normality of the quantitative variables. Nonparametric two-tailed tests were applied when necessary. For comparison of two means, the Student’s t- test and the nonparametric Mann–Whitney test were used. To verify the presence of a correlation between the variables, Pearson and Spearman correlations were used according to the normality of the data. The correlation coefficients can vary from -1 to +1 and are categorized as weak (r<0.3), moderate (r =0.3-0.7) or strong (r>0.7).22
Ethical considerations
This study was approved by the Ethics and Research Committee of the Federal University of Espirito Santo, under the number CAAE 27954014.0.0000.5060.
Seventy patients were evaluated. The mean age was 58.51±14.85years, 51.4%(n = 36) were elderly and female. Regarding the location of the cancer, the lower gastrointestinal tract was most affected, with 42.9%(n = 30) cases, followed by upper gastrointestinal cancer, present in 30.0%(n=21) of the patients. Bladder and soft tissue cancer were grouped in the other category (Table 1).
|
|
Transthyretin |
|
|
Variable |
N=70 |
>17,0mg/dl Without risk nutritional |
≤ 17,0mg/dl With risk nutritional |
p value |
Age |
58.51±14.85 |
56.48±16.71 |
60.54±12.64 |
0.256 |
Sex |
N (%) |
N (%) |
N (%) |
|
Male |
34(48,6) |
22(61.1) |
14(38.9) |
0.093 |
Female |
36(51.4) |
13(38.2) |
21(61.8) |
|
Life stage |
||||
Adult |
34(48.6) |
19(55.9) |
15(44.1) |
0.473 |
Elderly |
36(51.4) |
16(44.4) |
20 (55.6) |
|
Tumor location |
||||
Upper GI |
21(30.0) |
12(57.1) |
09(42.9) |
0.211 |
Lower GI |
30(42.9) |
16(53.3) |
14(46.7) |
|
Pancreas |
07(10.0) |
02(28.6) |
05(71.4) |
|
Bile ducts |
05(7.10) |
04(80.0) |
01(20.0) |
|
Lung |
03(4.30) |
- |
03(100.0) |
|
Others |
04(5.70) |
01(25.0) |
03(75.0) |
|
SGA |
||||
Well-nourished (A) |
20(28.6) |
14(70.0) |
06(30.0) |
0.66 |
Suspected/Moderate malnourished (B) |
23(32.9) |
08(34.8) |
15(65.2) |
|
Severe malnourished (C) |
27(38.6) |
13(48.1) |
14(51.9) |
|
Table 1 Sample distribution according to demographic variables, tumor location and nutritional status
GI, gastrointestinal tract; SGA, subjective global assessment
Figure 1 shows the prevalence of SGA malnutrition, serum transthyretin and serum albumin levels. SGA, transthyretin and serum albumin identified the presence of malnutrition in 71.56%(n=50), 50.0%(n=35) and 32.9% (n=23), respectively. There were no significant differences between them (SGA x transthyretin, p=0,237, SGAx albumin: 0.220).
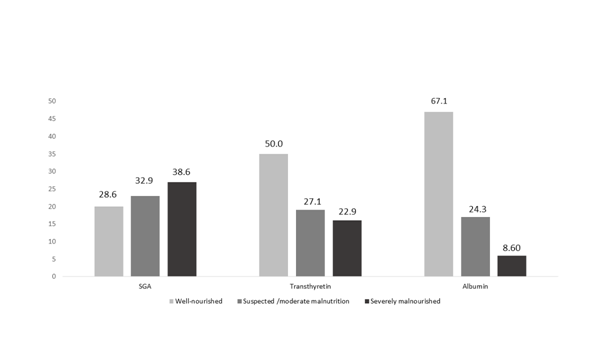
Figure 1 Prevalence of malnutrition in cancer patients from Global Subjective Assessment, serum transthyretin and albumin.
Table 2 shows the averages of the anthropometric and biochemical variables according to the groups with and without nutritional risk, classified from the values of transthyretin. Significant differences were found for AC variables (p=0.011) and CAMA (p=0.012). It is worth noting that CRP levels did not differ statistically between the groups with and without nutritional risk, suggesting that transthyretin levels were not influenced by inflammation.
|
Transthyretin |
||
Variable |
> 17,0mg/dl Without risk Nutritional |
≤ 17,0mg/dl With risk nutritional |
p value |
CC (cm)b |
33.71±3.89 |
28.49±4.18 |
0.173 |
AC (cm)a |
28.49±4.18 |
25.90±4.03 |
0.011* |
TSF (mm)a |
15.71±6,76 |
12.42±7.18 |
0.053 |
TAPM (mm)a |
14.90±4.40 |
14.10±5.39 |
0.494 |
CAMA (cm2)a |
37.50±13.33 |
30.59±8.71 |
0.012* |
ACM (cm)a |
23.37±3.63 |
21.90±2.52 |
0.076 |
BMI (kg/m2)a |
23.26±4.49 |
23.20±4.74 |
0.958 |
Albumin (mg/dL)a |
3.64±0.44 |
3.63±0,46 |
0.935 |
CRP (mg/dL)b |
23.35±29.37 |
39.02±52.51 |
0.344 |
Table 2 Comparison of anthropometric and biochemical variables according to transthyretin categories
aStudent T test; bMann-Whitney test
CC, calf circumference, AC, arm circumference; TSF, triceps skinfold; TAPM, thickness of the adductor pollicis muscle; ACM, arm muscle area; CAMA, corrected arm muscle area; BMI, body mass index; CRP, C-reactive protein. *p<0.05
Correlations between transthyretin and anthropometric and biochemical variables are described in Table 3. Although significant, there was a weak correlation between transthyretin and AC (p=0.019), while moderate correlations were found with ACM variables (p=0.008), CAMA (p=0.001) and albumin (p <0.00).
Variable(n=70) |
r |
p value |
CC (cm)a |
0.194 |
0.107 |
AC (cm)a |
0.286 |
0.025* |
TSF (mm)a |
0.022 |
0.857 |
TAPM (mm)a |
0.072 |
0.569 |
ACM (cm)a |
0.312 |
0.008** |
CAMA (cm2)a |
0.382 |
0.001** |
BMI (kg/m2)a |
-0.7 |
0.554 |
Albumin (mg/dL)a |
0.011 |
0.93 |
CRPb (mg/dL)b |
-0.116 |
0.34 |
Table 3 Correlation between serum transthyretin concentrations, anthropometric and biochemical variables
aPearson's correlation; bSperman correlation; *p<0,05*; **p<0,01; CC, calf circumference, AC, arm circumference; TSF, triceps skinfold; TAPM, thickness of the adductor pollicis muscle; ACM, arm muscle area; CAMA, corrected arm muscle area; BMI, body mass index; CRP, C-reactive protein
When compared to SGA, transthyretin exhibited sensitivity of 58.0% and specificity of 70.0%. The positive predictive value (PPV) was 83.0% and the negative predictive value (NPV) was 40.0%. The accuracy of the test was 61.0%.
The findings of the present study showed the association of transthyretin with the anthropometric variables used to evaluate nutritional status, especially those that evaluate the reduction of energy and protein reserves, such as AC, ACM and CAMA. Although transthyretin was not associated with SGA and serum albumin, a reduction in its concentrations was observed with the worsening nutritional status, indicated by categories B and C of SGA. Patients with cancer present with rapid weight loss and the presence of early malnutrition, not always occurring concomitantly. Weight loss may not be the first sign of malnutrition, which reaffirms the need for early markers of nutritional status, such as transthyretin.23
The decline of serum proteins is associated with a higher incidence of morbidity and mortality in cancer patients.24 Reduced levels of these proteins may be related primarily to nutritional changes, as well as being sensitive to nutritional intervention, since they have a high capacity for hepatic synthesis in the presence of protein intake.25,26 Unlike transthyretin, serum albumin is not able to assess acute changes in nutritional status due to its long half-life, which is more adequate for chronic malnutrition,27 corroborating the results of this study, in that normal albumin values were present even in the groups with low transthyretin levels.
As for anthropometric parameters, they are unable to detect recent disturbances of nutritional status, and therefore, when applied alone, do not reflect the patient’s actual nutritional status.28,29 Our results showed that despite significant results, AC, ACM and CAMA presented, respectively, a weak and moderate correlation with serum transthyretin levels. Thus, the use of transthyretin may be a complementary tool in the initial assessment of nutritional status.4,26,30
Transthyretin has also been recognized as a possible preoperative risk marker and infection,30 by anticipating the acute phase response and thus allowing the nutritional strategy to be traced at this stage.9
The hospitalized patient presents several clinical conditions that, in addition to malnutrition, affect serum proteins. Devoto et al.,26 found a correlation between nutritional status and transthyretin, independently of the concentrations of CRP, a condition observed in this study.
Although the majority of patients with nutritional risk have low levels of transthyretin, the low specificity attributes low diagnostic value to this tool. Diseases that cause proteinuria and exacerbate acute phase response, such as neoplasms, may be responsible for the increase in the false positive.31
Studies of patients diagnosed with head and neck cancer18 and esophageal cancer submitted to radiotherapy19 obtained significantly lower levels of transthyretin in malnourished patients, as defined by SGA, when compared to well-nourished patients. In contrast, albumin did not present a significant difference between nutritional status and between groups at the beginning and end of radiotherapy.
Given its limitations and applications, the use of transthyretin as a complement to the classic nutritional status assessment methods is suggested, with the aim of providing a more complete and careful evaluation, as well as to identify patients at nutritional risk early, and to plan and follow-up nutritional intervention.
Among the limitations of this study, it is emphasized that caution is required in the interpretation of biochemical tests as a function of factors such as perioperative fasting, hemodilution, renal diseases and tumor staging that may have affected levels of transthyretin in the blood. In addition, it should be noted that this study involved only cancer patients, a fact that limits the extrapolation of the results.
Transthyretin was moderately associated with the anthropometric variables arm circumference and corrected arm muscle area which represents the energy and muscle mass reserves. The results show the difficulty of using transthyretin in the diagnosis of malnutrition in the cancer patient, since it is influenced by several factors present in these individuals. However, transthyretin may be a complementary tool in the initial nutritional assessment, because it is feasible, easy to interpret and undergoes changes in a short period.
Special thank you goes to the University Hospital Cassiano Antônio Moraes for all support and assistance throughout the research and Health Sciences Centre/Federal University of Espirito Santo.
The author declares that there is no conflict of interest.

©2018 Barreto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.