Journal of
eISSN: 2373-633X


Research Article Volume 9 Issue 3
1Department of Radiology, The Seventh Affiliated Hospital, Sun Yat-sen University, China
2Department of Interventional Oncology, The First Affiliated Hospital, Sun Yat-sen University, China
Correspondence: Dr. Ying-Qiang Zhang, Department of Radiology, The Seventh Affiliated Hospital, Sun Yat-sen University, 628 Zhenyuan Road, Shenzhen, P.R. China, Tel 86-0755-81206512
Received: April 18, 2017 | Published: May 21, 2018
Citation: Di-Min L, Zhong-Xing L, Xing-Kui C, et al. The radiologic assessment of progressive disease for patients with hepatocellular carcinoma after transarterial chemoembolization should be stratified. J Cancer Prev Curr Res. 2018;9(3):108-110. DOI: 10.15406/jcpcr.2018.09.00331
Assessment ofprogression according to EASL or mRECIST after transarterial chemoembolization (TACE) has certain of limitations, mainly because of unspecified number of new lesions and primary tumor response was not considered. We retrospectively analyzed 105 HCC patients with progression after initial TACE. In the analysis, overall survival was significantly longer in subgroup with new lesions ≤3, primary tumor response, and decreased AFP than that with new lesions >3, non-primary tumor response, and increased AFP, respectively. Therefore, a comprehensive analysis of primary tumor response, along with number of new lesions and change of AFP, could make an accurate assessment.
Keywords: radiology, assessment, progressive disease, hepatocellular carcinoma, transarterial chemoembolization
The European Association for the Study of the Liver (EASL) and modified Response Evaluation Criteria in Solid Tumors (mRECIST) can reliably predict the treatment response and survival in patients with hepatocellular carcinoma (HCC) undergoing transarterial chemoembolization (TACE), and are widely used to assess the tumor response in patients with HCC after TACE.1 However, based on these imaging criteria, the treatment response was classified as having progressive disease (PD) if an unspecified number of new lesion or metastasis were detected; however, primary tumor response was not considered.2,3
Recently, database including HCC patients who were treated using TACE at our center were analyzed.4 Interesting, we found that a subgroup of patients with unresectable HCC and who were classified as having PD after TACE based on EASL or mRECIST criteria, have relatively longer survival times. In the present study, we defined the survival time as more than 12 months, which is significantly longer than nature survival rates (about 6 months) of patients with unresectable HCC.5 Generally, a patient who undergoes treatment and is classified as having PD indicates poor response to the method, subsequently leading to poorer survival rates. Therefore, we think this group of patients who were classified as having PD according to EASL or mRECIST should be divided into subgroups and analyzed. Specifically, the following characteristics were used: (a) the primary tumor has obtained a response to TACE, (b) the number of new lesions or metastasis was relative small, or (c) decreased alpha-fetoprotein (AFP) level. To validate this hypothesis, we conducted an analysis of a cohort of patients with PD after an initial session of TACE.
The study protocol was approved by the ethics committees of the institution. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. Between January 2012 and December 2014, a total of 105 consecutive patients with unresectable HCC classified as PD after initial TACE were collected for the analysis. According to EASL guideline, we defined patients with more than 3 new lesions as having multiple lesions.6 The all new HCC lesions were >1cm and met the characteristics for the imaging diagnosis of HCC (ie. arterial enhancement and washout on venous phases). Similarly, patients with AFP >20% increase in baseline is definitive of AFP progression according to a published article.5,7
The TACE procedure was performed using techniques described previously.4 Briefly, 10-20mL lipiodol (Guerbet, Paris, France) was mixed with 20–40 mg epirubicin (Pfizer, New York, USA) to create an emulsion. Depending on the tumor size and liver function, 2–20mL of the emulsion was infused into the liver tumor via a catheter. Subsequently, gelfoam embolization was performed. When blood flow slowed or a vascular cast was observed, the injection was discontinued. Depending on the tumor distribution, the lobar, segmental, or subsegmental tumor-feeding artery was targeted, preferentially. TACE procedure was carried out by clinicians who had more than 10 years of experience in TACE. The TACE treatment was followed an ‘on-demand’ strategy. Additional TACE was indicated if residual viable tumors or new lesions were evident on contrast-enhanced computed tomography (CT) images, and there was preserved liver function.
Standard follow-up evaluations protocol of treatment for HCC, including dynamic contrast-enhanced CT scans and laboratory tests, were performed to evaluate the efficacy at 4-6 weeks after initiation of therapy. Laboratory tests included hematologic analyses, liver function test, serum AFP assay, and hepatitis serologic test.
All statistical analyses were performed using SPSS software (SPSS version 16.0, Chicago, IL). The Kaplan–Meier method and log-rank test were used to calculate and compare survival differences. Cox proportional hazards model to identify risk factors associated with survival.
The baseline characteristics of all patients are shown in Table 1. The majority of patients were male with a mean age of 49 years, and hepatitis B virus and cirrhosis were the most common underlying disease. Most of patients (75.2%) were at Barcelona Clinic Liver Cancer (BCLC) stage C, and 100 patients (95.2%) were in Child-Pugh A class, and 80(76.2%) patients had Eastern Cooperative Oncology Group (ECOG) status of 1. The mean number of TACE procedures per patient was 2.1 (range, 1–5). The mean follow-up duration was 8.3 months (range, 3-32 months). At the end of follow-up, all of patients had died.
Characteristics |
Number |
Age (mean, SD) (years) |
49.7±11.5 |
Sex (male/female) |
100/5 |
Etiology (HBV/HCV/other) |
101/0/4 |
Cirrhosis (Yes/No) |
81/24 |
No of tumors (1-3/>3) |
28/77 |
Size of main tumor (cm) (<5/5-10/>10) |
7/35/63 |
ECOG (0/1/2) |
15/80/10 |
Child-Pugh A/B |
100/5 |
BCLC B/C |
26/79 |
PVTT (Yes/No) |
76/29 |
Extrahepatic spread (Yes/No) |
20/85 |
AFP (ng/mL) (≤20/>20) |
7/98 |
Table 1 AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; PVTT, portal vein tumor thrombus
In the survival analysis, the median overall survival (OS) for all groups (n=105) was 6.0(95% CI: 5.2-6.8) months. The median OS in patients with new lesions ≤3(n=35) was 11.0(95% CI: 9.7-12.2) months, whereas in those with new lesions >3(n=70), it was 5.0(95% CI: 5.1-6.8) months (HR: 0.25; 95% CI: 0.12-0.54; p<0.0001; Figure 1A). Furthermore, the median OS in patients with primary tumor response (n=34) was 11.0 (95% CI: 9.7-12.3) months, whereas it was 5.0(95% CI: 4.5-5.5) months (HR: 0.18; 95% CI: 0.08-0.38; p<0.0001; Figure 1B) in those with non-primary tumor response (n=71). Finally, the median OS was 6.0 (95% CI: 5.2-6.8) months for patients with AFP progression (n=57) and 10.0(95% CI: 8.4-11.6) months (HR: 0.19; 95% CI 0.11-0.31; p<0.0001; Figure 1C) for patients with AFP response (n=41).
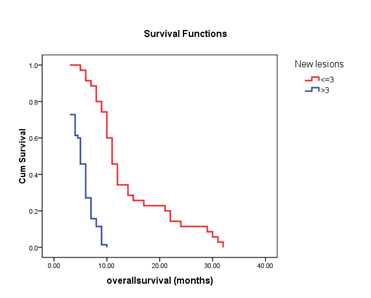
Figure 1A The median OS was 11.0 months for patients with new lesion ≤3 (n=35) and 5.0 months for patients with new lesion >3 (n=70) (log-rank test, p <0.0001).
There are a number of explanations for these findings. First, TACE potentially causes hypoxia in tumors as well as surrounding liver tissues because of the anti-cancer effects of chemotherapy and embolization of feeding arteries. Ischemic injury after TACE can induce upregulation of circulating vascular endothelial growth factor, which is essential for HCC growth, invasion, and metastasis.8 Therefore, the risk of metastasis, may increase following TACE. Patient with new lesions or metastasis ≤3 were classified as having oligometastatic cancer and can be managed according to HCC guideline.6,9 Consequently, this subgroup of patients underwent subsequent therapies to treat the primary and new metastasis lesions. Similarly, M. K Barton9 presented his comments in CA: A Cancer Journal for Clinicians in which he indicated that local consolidative therapy may be beneficial in patients with oligometastatic (three or fewer lesions) non-small cell lung cancer. Therefore, TACE is efficacious and may prolong the survival time of patients. On the other hand, when a patient appeared more than 3 (multiple) new lesions as a result of TACE, the patient is considered to have aggressive tumors or metastatic tumors. Patients with multiple new lesions exhibited poor outcomes. Second, the tumor biomarker AFP level can provide an objective reflection of tumor burden and activity10 Therefore, the change of AFP level may objectively reflect the overall changes in tumor burden. Although new lesion or metastatic lesions might have appeared as a result of TACE, the primary tumor might have been affected. This would therefore decrease the tumor burden, and, decrease or stabilize serum AFP. This confirmed the results of previous studies that showed AFP response may predict survival in HCC patients treated with TACE.7,10
The assessment of PD after TACE based on the EASL and mRECIST imaging criteria has certain of limitations, mainly because the primary tumor response was not accounted for. However, a comprehensive analysis of primary tumor response, along with the number of new lesions or metastasis and the change of AFP level (if necessary), could make an objective and accurate assessment.
We would like to thank Boning Luo (Department of Radiology, The Seventh Affiliated Hospital, Sun Yat-sen University, and Shenzhen) for imaging assistance.
All authors have declared no conflicts of interest.

©2018 Di-Min, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.