Journal of
eISSN: 2373-633X


Review Article Volume 8 Issue 4
1Center for Stem Cell and Regenerative Medicine, University of Texas Health Science Center at Houston (UTHealth), USA
2Center for Tissue Engineering and Aging Research, University of Texas Health Science Center at Houston (UTHealth), USA
3Department of Traumatic Surgery, Tongji Hospital, Tongji Medical College, China
4Center for Cancer Epigenetics, University of Texas, USA
5Department of Orthopaedic Surgery, McGovern Medical School, USA
6Center for Regenerative Sports Medicine, Steadman Philippon Research Institute, USA
7Department of Internal Medicine, McGovern Medical School, USA
Correspondence:
Received: August 10, 2017 | Published: August 22, 2017
Citation: Xiaojing D, Fan Y, Xiaobing S, et al. Potential mechanisms behind physical exercise vs. epigenetic regulation for preventing breast cancer. J Cancer Prev Curr Res. 2017;8(4):289-297. DOI: 10.15406/jcpcr.2017.08.00282
Breast cancer is the most commonly diagnosed cancer and has been the second leading cause of death due to cancer among women worldwide. It is a disease in which malignant (cancer) cells form in the tissues of the breast and become deadly when they metastasize, spreading to other organs or bones. Breast cancer is one of the major cancer diseases, occurring in both men and women, but it is rare in men. Though risk for breast cancer is affected by genetic factors, age, and other factors or personal habits; recent studies have suggested that physical exercise induces positive effects for lowering these risks as well as reducing the incidence rate. In the current review, we will look at previous studies, which have investigated links between physical exercise and breast cancer in the last decade. Meanwhile, we will discuss potential mechanisms by which physical exercise may play a role in preventing breast cancer, with special emphasis on a recent study on epigenetic regulations during physical exercise.
The incidence of patients with breast cancer is increasing every year. Breast cancer has recently been ranked as the second most common cancer in women, just after skin cancer in the U.S. However, death rates from breast cancer have been declining since 1990, due in part to better screening and early detection, increased awareness, and continually improving treatment options. In recent years, new applications in both diagnosis and treatment have gradually reduced female breast cancer incidence rates.1 Considering alternative prevention and therapeutic methods, physical exercise has recently been gaining increased attention in the clinic. Regular exercise appears to lower breast cancer risk by about 10-20 percent, which is a considerable impact from a non-pharmacologic intervention with no cost. Thus, the recently emerging field of exercise-oncology research will be expected to not only clarify the mechanisms behind prevention, but also shed light on future treatment for breast cancer. In this current review, we will summarize recent studies on physical exercise with respect to breast cancer, and discuss their relationship and possible underlying mechanisms.
Risk factors for breast cancer
Breast cancer is the second most common type of cancer and the leading cause of cancer-related death in women worldwide (link: https://www.breastfriends.org). It is a tremendous disease, affecting lifespan and healthspan. The incidence of breast cancer is increasing every year, and an estimated 252,710 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S. in 2017 (link: http://www.breastcancer.org/symptoms/understand_bc/statistics). Throughout the world, every year more than 400,000 deaths are caused by breast cancer and, out of these diagnosed cases, there is more than 2.8 million breast cancer survivors.2
Generally, there are two aspects of risk factors for breast cancer (see Table 1): (1) non-modifiable risk factors, which cannot be changed, such as gender.3,4 age5-8 race and ethnicity,9,10, national development11-14 family history/biopsy.15-18 menstrual history,19-22 breast features23-25 blood type,26 personal disease history27-28 and genetic inherited changes29 and (2) modifiable risk factors, such as reproductive history,30-33 breast feeding,34-38 exogenous hormone exposure39,43 radiation exposure44,45 delay in diagnosis46 operation or treatment,47-54 oral agents55-57 and lifestyle.58-67 There have been extensive studies indicating that avoidance of cancer modifiable risk factors may be helpful for preventing breast cancer incidence and mortality.68-70 On the other hand, due to genetic susceptibility influences, which are inherited and unchangeable; the non-modifiable factors are not easily controllable risks for breast cancer. Therefore, it is feasible to reduce the risk factors that are not inherited, but can be changed. Among these modifiable risk factors, physical exercise behaviors not only are beneficial for preventing all stages of cancer development71 but also are helpful to maintain a healthy weight and reduce risks for developing other diseases, such as cardiovascular disease, diabetes, and stroke.72 Furthermore, physical activity (PA) has been increasingly regarded as the non-pharmacologic intervention for many cancer patients to combat both the physiologic and psychological effects of cancer treatment.73 A recent World Health Organization (WHO) report shows that physical inactivity remains one of the most important risk factors for total cancer related mortality (responsible for 6% of total deaths) along with overweight and obesity (responsible for another 5%).74
|
Non-modifiable risk |
Influence |
Modifiable risk |
Influence |
||
|
Female |
↑↑↑ |
Reproductive history.29-32 |
First full-term pregnancy at age 35 or older |
↑↑ |
|
|
Age4-7 |
>30 |
↑ |
|
Nulliparous |
↑↑↑ |
|
|
>40 |
↑↑ |
|
Periparous or multiparous at a younger age (before 20) |
↓ |
|
|
>50 |
↑ |
|||
|
|
>70 |
↑↑↑ |
|
Abortion |
- |
|
Non-Hispanic (white) |
↑↑↑ |
Breast feeding 33-37 |
Breast-fed |
↓ |
|
|
Non-Hispanic (black) |
↑↑ |
|
Breast-feeding |
↓↓ |
|
|
|
Alaska Native and American Indian and Hispanic |
↑ |
|
Never breast-fed |
↑ |
|
National |
Higher developed (U.S., Northern and Western Europe, Australia, and New Zealand) |
↑↑↑ |
Exogenous hormone exposure38-42 |
HRT in pre-and post-menopause |
↑ |
|
|
Intermediate developed (Central Europe, Latin America, and the Caribbean) |
↑↑ |
|
Oral contraceptives |
- |
|
|
Low developed (Africa and Asia) |
↑ |
|
Fertility drugs |
- |
|
Family history of breast cancer or biopsy14-17 |
First-degree relative (mother, sister, or daughter) |
↑↑ |
Lifestyle57-66 |
Exercise (more than 4 hours per week) |
↓ |
|
Breast feature22-24 |
Degree of density |
↑↑ |
|
Obesity |
↑ |
|
|
Degree of size |
↑ |
|
Alcohol |
↑ |
|
Blood type25 |
Type A |
↑ |
|
Smoking |
? |
|
|
R factor |
- |
|
Night shift |
↑ |
|
Menstrual history 18-21 |
Early menarche at 11 or younger |
↑ |
|
Diet |
? |
|
|
Menopause at a later age |
↑↑ |
|
Bra wearing |
- |
|
Benign (non-cancer) breast disease |
↑ |
|
Environment pollution |
- |
|
|
|
Viral warts (Human Papilloma Virus) |
↑ |
|
Underarm deodorants/ antiperspirants |
- |
|
|
Diabetes |
↑ |
Chest radiation therapy |
↑ |
|
|
Genetic inherited changes28 |
BRCA1 and BRCA2 gene mutations |
↑ |
|
Radiation during puberty |
↑↑ |
|
|
KRAS |
↑ |
Delay in diagnosis45 |
Developing country |
↑ |
|
|
Reproductive factors |
↑ |
|
Post-disaster |
↑ |
|
|
|
|
Operation or treatment46-53 |
Prophylactic |
↓ |
|
|
|
|
|
Prophylactic |
↓ |
|
|
|
|
|
Estrogen-only hormone therapy after hysterectomy |
↓ |
|
|
|
|
|
Selective estrogen receptor modulators (SERMs) |
↓ |
|
|
|
|
|
Aromatase inhibitors and inactivators |
↓ |
|
|
|
|
|
Implant |
- |
|
|
|
|
Oral agent54-56 |
Vitamins |
- |
|
|
|
|
|
Statins |
- |
|
|
|
|
|
Bisphosphonates(or intravenous infusion) |
- |
Table 1 Risk Factors for Breast Cancer
↑ upgrade; ↓ downgrade; - no influence; ? controversial.
Breast cancer and physical activity (PA)
Physical exercise brings many benefits to our healthy and non-healthy populations. Frequency of exercise not only directly plays a significant role in the prevention of breast cancer (incidence rate), control of breast cancer progression, and improvement of patient physical abilities and balance; but also indirectly minimizes fatigue and nausea and improves self-esteem and quality of life in breast cancer patients.75 Numerous epidemiologic studies have documented that PA can down-regulate factors that increase the risk of breast cancer, thus reducing incidence and mortality of breast cancer.76-79 In this review, we have summarized a collection of various population-based studies on breast cancer and PA, and compared the beneficial effect of exercise on cancer such as the cancer type, duration, intensity, and timing of exercise (before diagnosis, after diagnosis, or during treatment) (Table 2).76-92
Type of exercise |
Sample |
Timing of exercise |
Relationship with BREAST CANCER |
Reference |
Regular exercise |
a population-based survival study among 1,364 breast cancer cases within the Norwegian Counties, study was conducted during 1974-2005 |
before diagnosis |
women with BMI<25kg/m2 had a 66% reduction in overall mortality |
Emau et al., 2010 |
Physical activity (PA) |
a total of 126 women completed the study (60 control/66 intervention, 61 Hispanic/65 non-Hispanic) |
during treatment |
intervention group had a better impact during PA (P = 0.03) than controls |
K. Crew et al., 2016 |
PA |
4,643 women diagnosed with invasive breast cancer after entry into the Women's Health Initiative study of postmenopausal women |
before diagnosis and after diagnosis |
PA had lower all-cause mortality both pre-and post-diagnosis |
Melinda L. Irwin et al., 2011 |
Recreational PA |
1,264 women ages 20 to 54 years who were diagnosed with invasive breast cancer between 1990 and 1992 |
at the time of diagnosis |
reduced mortality among women who were overweight or obese (BMI>25kg/m2) |
Page E. Abrahamson et al., 2006 |
PA |
using prospective studies published through June 2013 |
before and after diagnosis |
PA performed pre-/post-diagnosis is related to reduced mortality risk among breast cancer |
M. F. Leitzmann et al., 2013 |
Recreational PA |
a prospective cohort study in Germany, including 3,393 non-metastatic breast cancer patients aged 50-74 years were assessed |
before diagnosis |
inverse association of pre-diagnosis recreational PA with mortality and recurrence of breast cancer patient |
Karen Steindorf et al.,2013 |
PA |
8 studies (12,108 patients with breast cancer) were included in this meta-analysis |
after diagnosis |
inverse relationship between PA and mortality with breast cancer |
Abdelaziz AlHomaidh et al., 2011 |
Recreational PA |
population-based sampling in Northern California, USA; Ontario, Canada; Melbourne and Sydney, Australia between 1991 and 2000 |
before diagnosis |
recreation PA was associated with lower risk of death with estrogen receptor (ER)-positive, but wasn't associated with all-cause mortality |
Esther M. John et al., 2010 |
Regular exercise |
within Norwegian Counties, study during 1974-2005 |
before diagnosis |
women with BMI<25kg/m2 and age of diagnosis >55 years had a 66% reduction in overall mortality |
Inger Thune et al., 2010 |
Consistent long-term participation in PA |
California Teachers Study Cohort members provided information in 1995-1996 on long-term tracking |
before diagnosis |
moderate and strenuous recreational PA before breast cancer may lower risk of breast cancer death |
Huiyan Ma et al., 2009 |
Both moderate and vigorous intensity recreational activity |
a cohort of 1,231 women diagnosed with breast cancer between 1995 and 1997conducted in Alberta, Canada |
after diagnosis |
both moderate and vigorous intensity recreational activity decrease the risk of breast cancer death |
Kerry S. Courneya et al., 2009 |
PA |
933 women diagnosed with local or regional breast cancer between 1995 and 1998 |
after diagnosis |
increased PA of breast cancer women had a 45% lower risk of death |
Leslie Bernstein et al., 2008 |
PA |
patients participants were cases in Wisconsin, Massachusetts, and New Hampshire between 1988 and 2001 diagnosed with invasive breast cancer |
after diagnosis |
overall mortality and mortality from breast cancer in women with breast cancer is reduced in women engaged in PA |
Walter C. Willett et al., 2008 |
Long-term athletic training |
5,398 living alumnae; 2,622 of whom were former college athletes and 2,776 were non-athletes |
before diagnosis |
Long-term athletic training may lower the risk of breast cancer |
M. Marguglio et al., 1985 |
PA |
2,246 women, including 1,238 women with histologically-confirmed breast cancer and age-matched controls without breast cancer from Pakistan |
after diagnosis |
Lacking in PA and post-menopausal status were found to have significant positive associations with breast cancer |
Hamid Rashid et al., 2016 |
Physical self-management interventions |
A total of 13 RCT'S representing 2,180 participants |
during treatment |
physical self-management interventions during breast cancer treatment as well as after the primary treatment seem to generate beneficial effects on quality of life |
Nick Gerbruers et al., 2016 |
6-month exercise program |
85 women for early-stage breast cancer (stage I to III) |
after diagnosis |
a reduction in depressive symptoms and lower leukocyte, neutrophil, and lymphocyte counts compared to control group |
Robert E. Coleman et al., 2014 |
Supervised exercise and home exercise group |
60 female breast cancer patients |
after diagnosis |
decrease in interleukin-8 and neutrophil-activating protein-78 levels in home exercise |
Izmir & R. USLU et al., 2013 |
Table 2 Summary of Breast Cancer and Physical Activity Studies
Numerous studies have suggested that PA prior to breast cancer diagnosis was significantly beneficial for the patient’s treatment outcome and survival.76-92 For example, a group of researchers performed a prospective cohort study in Germany which included 3,393 non-metastatic breast cancer patients: the mortality of breast cancer was significantly and inversely associated with pre-diagnosis recreational PA.77 In addition, another similar study from Dr. Gammon and his colleagues demonstrated recreational PA prior to breast cancer diagnosis can improve patient survival.93 The cancer type, timing, and intensity of the PA are also important and should be taken into consideration. According to the 2008 Physical Activity (PA) Guidelines, it is recommended to engage in at least 2.5 hours per week of moderate-intensity PA (10 metabolic equivalent task (MET)-hours/week, defined as 4 METs) to reduce the risk of cancer related morbidity and mortality. An enhanced epidemiological study from Jeannette and colleagues evaluated 13,000 breast cancer survivors in the After Breast Cancer Pool Project (ABCPP) from multiple cities in the U.S. and from Shanghai, China.94 After 18-48 months post-diagnosis, they concluded that engaging in at least 10 MET-hours/week of PA was associated with a 27% reduction in all-cause mortality and a 25% reduction in breast cancer mortality. Among these women, 1,508 had an initial breast cancer diagnosis between 1996 and 1997.94 and their ratio of physical activity (RPA) was classified according to their MET. A lower risk of all-cause death was observed for women who were engaged in an average of 9 or more MET-hours/week of PA, from menarche to diagnosis, when compared with women who did not exercise. This was also observed in menopausal status.93 More recently, results from a systematic review and random-effects meta-analysis for pre- and post-diagnosis PA in relation to breast cancer mortality were reported.95 By Nov 2012, there were 31 studies with 63,786 cases reported with meta-analysis, and results suggested that the intensity of PA is strongly associated with risk of breast cancer-related death. Additionally, 34 randomized controlled trials assessed the effects of PA using meta-analysis, and indicated that PA has a beneficial effect on the physiology, body composition, physical functions, psychological outcomes, and quality of life in patients after treatment for breast cancer.96
On the other hand, the studies also showed that lack of PA is associated with a higher breast cancer occurrence. A population-based sampling in the U.S., Canada, and Australia was investigated with follow-up for 7 - 8 years, and showed that patients engaging in recreational activity during the 3 years prior to diagnosis had a 34% lower risk of cancer-related death. It was estimated that about 7.9% of Canadian patient cancer cases (breast, colon, endometrium, prostate, lung, and ovarian) were associated with physical inactivity, demonstrating that thousands of cancer cases may be prevented by following a healthy lifestyle which includes PA.85 In a study from Norwegian Counties spanning from 1974 to 2005, 1,364 breast cancer patients were surveyed. Women with a body mass index (BMI) of <25 kg/m2 and an age of diagnosis of >55 years had a 66% reduction in overall mortality if they exercised regularly before diagnosis compared to sedentary women.80 Another study from the California Teachers Association reported that patients engaging in high or intermediate levels of long-term PA had a lower risk of breast cancer-related death (RR, 0.53; 95%CI, 0.35-0.80) than patients with low activity levels (RR, 0.63; 95%CI, 0.45-0.93). However, there are some reports that have suggest PA is not significantly associated with breast cancer mortality. An Italian multicenter, case-controlled study suggested that there was no significant relationship between survival after breast cancer emergence and several major lifestyle factors, including PA.97 These controversial results may be due to variations in the amount of PA and duration. One report from Irwin et al. found that women who participated in moderate-intensity recreational PA, such as brisk walking, post-diagnosis had a 64% lower risk of cancer-related death than inactive women.78 Hence, physical activity may not only lower the risk of breast cancer incidence but can influence survival after breast cancer diagnosis.77,97,98
Potential mechanisms behind physical exercise for preventing breast cancer
Physical exercise has been reported to have the potential to reduce risk of death from all stages of carcinogenesis (initiation, promotion, and progression).71 Previous studies have discussed that physical exercise may affect tumor initiation by enhancing the cytochrome P450 system and enhancing selective enzymes in the carcinogen detoxification pathway, such as glutathione-S-transferase.99 In addition, physical exercise may also prohibit promotion and progression stages of carcinogenesis by scavenging reactive oxygen species (ROS);100 altering cell proliferation, apoptosis, and differentiation;71 decreasing inflammation;101 and enhancing immune function and suppressing angiogenesis.99 With this complex information, we will sum up previous reports and discuss potential mechanisms behind the relationship between PA and breast cancer in several aspects, from whole body and the metabolic system to molecular pathways(Figure 1).102-105
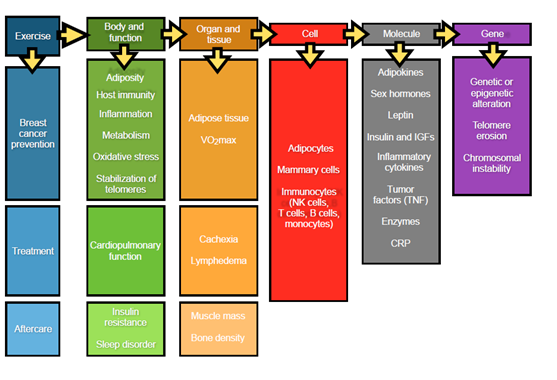
Figure 1 Potential mechanisms behind the relationship between PA and breast cancer in several aspects, from Whole body and the metabolic system to molecular pathways.
Inflammatory reactions
Treatment of cancer is related to disruption of immune system functions.106,107 Some studies have investigated whether exercise can modulate circulating proinflammatory cytokines, such as IL-1β, IL-2, IL-4, macrophage inflammatory protein-1 beta (MIP-1β), and tumor necrosis factor alpha (TNF-α),92,108,109 suggesting a protective effect of exercise on inflammation perturbation following cancer treatment. A systematic review suggests that physical exercise can lead to changes in a variety of immune system parameters, including lymphocyte function such as improved cytotoxic activity by natural killer (NK) cells and proliferation of granulocytes.110 Moreover, Jones and his colleagues illustrated aerobic training cause’s favorable alterations in metabolic and immune-inflammatory pathways.111 A more recent study showed that exercise training and tamoxifen reduced tumor IL-6 levels as well as NF-kB and STAT3 expression, and up-regulated TPM1 and PDCD4 expression (P<0.05).112 Rogers et al. reported that exercise reduces pro-inflammatory serum cytokines through changes in body composition and, in turn, attenuates fatigue, improves sleep quality, and reduces the risk of cancer recurrence.113
There are controversial results among these studies with respect to immune system responses during or after exercise. It is possible that various results come from differences in physical exercise intensity and duration, as well as heterogeneous patient populations and differences in study methodology.114 However, it has been gradually accepted throughout the research community that the effects of physical exercise are beneficial to the immune system and its defense mechanisms with respect to cancer physiology and pathology processes. A previous study indicated that some acute exercise-induced factors, such as IL-6 (interleukin 6), NGPTL-4 (angiopoietin like 4), MCP-1 (monocyte chemotactic protein-1 also known as CCL-2), CX3CL1 (fractalkine), IL-8 (interleukin 8), IL-15 (interleukin 15), Irisin, and secreted protein acidic and rich in cysteine (SPARC) are produced locally in skeletal muscles during muscle contraction, and can be released into arterial blood. Furthermore, it has been reported that muscle-derived oncostatin M (OSM) and Irisin can decrease breast cancer cell viability, while SPARC inhibits tumorigenesis in the colon of exercising mice.115 Herein, we have summarized the effects of physical exercise on other aspects of the body and overall health, such as inflammatory adipose tissue metabolism103 sex hormones116 leptin102 and tumor factors,117 cardiopulmonary function,118-120 insulin resistance and sleep disorders120 cachexia and lymphedema2, muscle mass and bone density,121 and genetic alteration(Figure 1).122
Epigenetic regulation
Beyond the above-described metabolic impact of physical exercise on breast cancer, an emerging field of exercise-oncology research is poised to determine the efficacy of, and biological mechanisms by which, aerobic exercise affects cancer incidence, progression, and/or metastasis.123 However, it is not clear what molecular mechanisms are involved and how physical exercise prevents or reduces incidence and mortality of different kinds of cancers. There is growing evidence suggesting that traits of trainability (the response to exercise training) might depend on epigenetics. Environmental effects, such as nutrition, diet, and exercise can alter epigenetic marks from either DNA methylation or post-translational modifications (acetylation, phosphorylation, and ubiquitination) of histones.124 One particular study suggests an indirect link between exercise and cardiovascular diseases through epigenetic modulation.124 It has also been reported that the myogenic genes in human skeletal muscle are altered by physical activity.125 In addition, it has been found that epigenetic regulation of the tumor suppressor gene, L3MBTL1, has a favorable impact on survival outcomes of breast cancer patients.126 However, how physical exercise affects oncogenes through alternating epigenetic regulation remains a question. It has been discovered that exercise results in aberrant DNA methylation patterns in tissues that have been extensively described as triggers for carcinogenesis.127 It has also been suggested that exercise and epigenetics activate an intricate mechanism that induces metabolic adaptation.72 Shock and colleagues also reported a possible exercise-induced methylation effect in mitochondrial genomes.128 At current, there are still controversial reports and discussions of the association between PA and DNA methylation.129-131 Therefore, it will be important to investigate the essential roles of epigenetic factors and regulation with respect to physical exercise and health benefits. Furthermore, certain challenging concepts need to be clarified, including how much the intensity and duration of physical exercise impacts the benefits of PA to provide the best effects on health, prevent aging, and decrease the incidence of cancers.
There are various mechanisms possibly associated with the benefits of exercise on epigenetic regulation in cancer prevention or treatment. Recently, ASC (apoptosis speck-like protein containing a CARD (caspase recruitment domain)) was investigated in apoptosis and tumor suppression. The gain- and loss-of-function of ASC in the study indicated an effect of promoter methylation on the regulation of TMS1 (target of methylation-induced silencing) in breast cancer and its essential role in docetaxel chemotherapy, suggesting potential benefits of ASC associated with cancer chemotherapy.132 From this study, compared to an older control (no exercise) group, the degree of ASC methylation was higher in an older exercise group with presumably lower ASC expression. Though chronic moderate exercise was also reported to modulate pro-inflammatory cytokines in the previous study, the detailed mechanism behind ASC in cancer is still under investigation. It has been reported that chronic moderate exercise appears to attenuate the age-dependent decrease in ASC methylation, implying there is suppression of excess pro-inflammatory cytokines through reduction of ASC expression.133 Beyond the protein alteration in tumor apoptosis and suppression, the physical stimulation/exercise of skeletal muscle also plays a critical role in mediating potential health benefits.117-120 Although the precise mechanism is not clear, chromatin remodeling through epigenetic histone modification has appeared as a critical regulatory mechanism that controls gene expression in general.125 Other accumulating data suggest that exercise of moderate intensity causes regulation of redox homeostasis and signaling, including generation of appropriate ROS levels.99,124 It is still unknown whether these factors might influence tumor progression, apoptosis, and metastasis. Exercise can also promote angiogenesis and knowing that the blood vessel walls contain a variety of stem cells (perivascular endothelial and mural cells).134-136 and some of these stem cells have recently been shown to have an inhibitory effect on cancer cells through unknown mechanisms, the beneficial effect of exercise on cancer development and prevention is maybe related to these blood vessel derived cells.
Breast cancer accounts for 75% of all cancers and 15% of cancer-specific deaths amongst females. However, death rates from breast cancer have been falling year by year, mainly due to new technology for early detection and therapeutic development. Physical exercise is beneficial to breast cancer patients through all stages (prevention, diagnosis, and survival) via immune response mechanisms, metabolism, hormone regulation, insulin resistance, anti-oxidant enzymes, and epigenetic regulation. Another hot topic is investigation of biomarkers, which plays an essential role in the management of patients with invasive breast cancer. Biomarkers and diagnostic tools, such as Kir67, urokinase plasminogen activator (uOA)-PAI-1, Oncotype DX, MammaOrint, EndoPredic, Breast Cancer Index (BCI) and Prosigna (PAM50) may be used to predict outcome and aid in adjunct therapy according to guidelines from the European Group on Tumor Markers.137,138 Frequent exercise not only stimulates or modifies changes in both genes and proteins that are biomarkers for diagnosis of breast cancer, but also reduces risk factors and is associated with successful treatment outcomes for breast cancer. Therefore, physical exercise is capable of bringing the benefits of both prevention and treatment in breast cancer, and increases the survival of breast cancer patients.
The authors would like to thank Dr. Mary Hall for her excellent editing of this manuscript.
The authors declare that there is no conflict of interests regarding the publication of this paper.
None.

©2017 Xiaojing, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.