Journal of
eISSN: 2373-633X


Research Article Volume 8 Issue 3
1University of Birmingham, UK
2Consultant Physician, New Cross Hospital, Royal Wolverhampton Trust, England
3Medical Faculty, Black Lion Hospital, Addis Ababa University, Ethiopia
Correspondence: Dominic Worku, University of Birmingham, 14 Cwrt-Ucha Terrace, Taibach Port Talbot Sa13 1ld, United Kingdom, Tel 7780663345
Received: June 12, 2017 | Published: August 4, 2017
Citation: Worku D, Raghavan RP, Tigeneh W, et al. What are the clinicopathological features and outcomes of sporadic colorectal cancer (CRC) in an ethiopian cohort with focus on young-onset CRC? J Cancer Prev Curr Res. 2017;8(3):253-258. DOI: 10.15406/jcpcr.2017.08.00276
Sporadic Colorectal cancer (CRC) is the 3rd and 4th most common cancer worldwide in women and men respectively and is responsible for over 550,000 deaths per year.1,2 While the incidence of this disease is rising, mortality for this condition has improved greatly from previous decades through the use of improved diagnostic criteria, awareness of risk factors, treatment modalities and screening led reductions in advanced/emergency presentations.3,4
Of this Global rise in CRC developing nations are playing an increasingly larger role. This is thought to reflect their westernization of their diet and lifestyle in addition to baseline genetic risk.5. Such changes and the metabolic syndrome they predispose to are key risk factors in CRC pathogenesis and the adenoma-carcinoma sequence taking on average 10-15years.6,7 In western societies, 90% of CRC cases affect patients >55years-old, however, it is becoming increasingly apparent that ‘young-onset CRC’ which is generally defined as ≤40years (although alternative definitions exist), is becoming more prevalent in developing and developed nations.1,8 Traditionally, young-onset CRC make up 7% of western cohorts, however, this figure is complicated by the inclusion of patients with predisposing genetic syndromes (e.g. Lynch Syndrome) and comorbid illness (e.g. Inflammatory Bowel Disease).9 In addition most observations regarding young-onset CRC have been made using Surveillance Epidemiology and End Result Registries (SEERs) based on American and World Health Organisation (WHO) databases. Given these factors and the rarity of such cases relevance of young onset CRC and interpretation of epidemiology in third world cohorts remains debatable.
Early onset sporadic CRC differs from traditional CRC (associated precursor adenomatous polyps or a family history) and it remains to be seen whether there are significant clinical, biological and outcome related differences in the former.10 A recent systematic review has reported profound differences between the presentations of early versus traditional-onset CRC. These include a greater incidence of adverse prognostic factors such as mucinous/signet ring histology, venous and perineural invasion and higher tumour stage and grade resulting in worse outcomes.11 Furthermore, young-onset patients may have a predominance for left-sided/rectal malignancy and not the rightward shift in CRC distribution observed in traditional-onset populations as a result of screening protocols.12,4 Nevertheless findings between studies regarding young-onset disease are inconsistent partly due to the different definitions regarding what constitutes ‘young’ as well as infrequent reporting of such cases. As such there may be no real difference in the histopathological features or the survivability of this tumour type, but this must be explored given its increasing incidence. Currently treatment regimens for CRC do not discriminate by age of disease onset but are instead informed by patients’ functional status, age, tumour characteristics, comorbid disease and choice.13
The aim of this study was therefore, to evaluate the epidemiology of CRC in Addis Ababa, Ethiopia to confirm if young-onset sporadic disease is indeed prevalent, and furthermore relate its clinicopathological characteristics and outcomes with traditional traditional-onset disease.
The Tinkur Anbessa Hospital in Addis Ababa is a state run hospital and the sole cancer referral centre in Ethiopia and at present maintains the only known cancer registry. Patients who had presented within the last 9 months were identified using the oncology admission and follow-up registry. Data from identified sporadic CRC cases was collected via patient notes and discussions with the multidisciplinary team (MDT). Due to multiple coding formats only cases specifying the exact diagnosis or tumour location were accepted. All patients with suspected or confirmed hereditary CRC syndrome were excluded from the study.
All patients had been seen independently by the hospital’s MDT, and in all cases a diagnosis of CRC was confirmed through colonoscopy and biopsy by a pathologist. As no onsite pathology/laboratory department exists, the diagnosis, biochemistry and Carcinoembryonic Antigen (CEA) measurements were conducted by several outside private sources. Young-onset CRC was defined as ≤40years old with those >40years old considered traditional-onset, these cut-offs were used in all analyses. In all cases details regarding duration of symptoms, mode and nature of presentation, co-morbid illness, baseline Eastern Cooperative Oncology Group (ECOG) status, family history, tumour histopathology, management aims, treatments offered and outcomes of these interventions were sought. Mode of presentation was defined as elective if surgery and treatment was planned and arranged, or, emergency if patient underwent hospital admission for complications including large bowel obstruction, perforation or life threatening haemorrhage. Follow up of all patients was performed in order to identify recurrence rates. Tumour staging was recorded using the American Joint Committee on Cancer Staging System (AJCC) which supersedes Dukes Classification.14 Right-sided lesions were defined as those from the cecum up to and including the hepatic flexure with transverse colon lesions recorded separately. Left-sided lesions included the splenic flexure and the descending colon but not the rectosigmoid junction. In cases of synchronous tumours, patients would be classified as multiple and not according to individual tumour location. Decisions on outcomes including stage of management (e.g. whether in remission or having progression etc) were based on whether treatment was curative or palliative in nature and were determined by discussions within an MDT setting.
Permission for this study was granted by the head of the Oncology department Dr Tigeneh Wondemagagnehu and the Dean of the University of Addis Ababa Medical School, Dr Daniel Siefu.
A total of 95 CRC cases were identified during the study period of which 85 were eligible for analysis (age range 16-75) with the remainder excluded due to coding errors (Table 1). 44 patients were ≤40years old (young-onset) with 73% between 30-39years and 41 patients >40years old (traditional-onset) with a Male: Female ratio of 1:1 and 1.41:1 respectively. Results are reported here as young/traditional-onset disease. Pathological staging was reported in 94% (n=80) with pT3 staging the most commonly reported (50%/51%) with 30% of young-onset cases exhibiting perineural/lymphovascular involvement. Appropriate nodal Assessment (>12 nodes) at biopsy was low having been performed in only 59%/56% of patients. Clinically, young-onset patients were more likely to present within 6 months of symptom onset (53%v39%) with 16% traditional-onset CRC patients presenting ≥24 months (range 24-39 months). Comorbid illness featured in 76% and 55% of traditional-onset and young-onset cases respectively. Of traditional-onset patients 33%(n=10) had features of the metabolic syndrome while 15%(n=3) young-onset had HIV. Performance status in the majority was ECOG 0/1 (77%/83%) with young-onset patients more likely to suffer functional limitation and were the only cases of ECOG V recorded (n=2). Family History featured in 9% and 5% of young and traditional-onset patients respectively however in the majority of individuals no history was recorded (52%v51%). In all patients with positive family history the presence of hereditary cancer syndromes was excluded.
All CRC Patients (n=85) |
young-onset CRC (n=44) |
traditional-onset CRC (n=41) |
|
Age Mean (years) |
43.6 |
33.2 |
55.2 |
29-Oct |
12 (13) |
12(27) |
0(0) |
30-49 |
43 (51) |
32(73) |
11(27) |
50-69 |
26(31) |
0(0) |
26(63) |
70+ |
4(5) |
0(0) |
4(10) |
Gender (%) |
|||
Male |
46(54) |
22(50) |
24(59) |
Female |
39(46) |
22(50) |
17(41) |
Region (%) |
|||
Addis Ababa |
56(66) |
24(55) |
32(78) |
Oromiya |
14(16) |
7(16) |
7(17) |
Snnpr |
6(7) |
5(11) |
1(2.5) |
Other |
9(11) |
8(18) |
1(2.5) |
Stage at Diagnosis (%) |
|||
T1 |
2(2) |
1(2) |
1(2) |
T2 |
12(14) |
6(14) |
6(15) |
T3 |
44(52) |
23(5) |
21(51) |
T4 |
22(26) |
13(30) |
9(22) |
N/A |
5(6) |
1(2) |
4(10) |
Nodal Involvement (%) |
|||
NX |
36(42) |
18(41) |
18(44) |
N0 |
17(20) |
8(18) |
9(22) |
N1 |
13(15) |
9(20) |
4(10) |
N2 |
8(9) |
6(14) |
2(5) |
N3 |
1(1) |
0(0) |
1(2) |
N4 |
3(4) |
2(5) |
1(2) |
N/A |
6(7) |
1(2) |
5(12) |
Symptom Duration(%) |
|||
0-6 months |
38(45) |
23(52) |
15(37) |
6-12 months |
18(21) |
7(16) |
11(27) |
12-24 months |
16(19) |
10(23) |
6(15) |
24+ months |
9(11) |
3(7) |
6(15) |
Unknown |
4(4) |
1(2) |
3(7) |
Presence of Co-morbid Illness (%) |
|||
Yes |
51(60) |
20(45) |
30(73) |
No |
34(40) |
24(55) |
11(27) |
ECOG status (%) |
|||
0 |
9(11) |
2(5) |
7(17) |
I |
59(69) |
32(72) |
27(66) |
II |
8(9) |
5(11) |
3(7) |
III |
3(4) |
1(2) |
2(5) |
IV |
0(0) |
0(0) |
0(0) |
V |
2(2) |
2(5) |
0(0) |
N/A |
4(5) |
2(5) |
2(5) |
Family History (%) |
|||
Yes |
6(7) |
4(9) |
2(5) |
No |
35(41) |
17(39) |
18(44) |
N/A |
44(52 |
23(52) |
21(51) |
Table 1 Baseline characteristics of 85 CRC patients divided by age as young (≤40years old) (n=44) and Late (>40 years old) onset (n=41).
were noted (5%v4%).
Tumour histopathology
All patients had histological data reported with adenocarcinoma the most common histological subtype (84%v85%) of which a history of polyps was only recorded in 3.5% of all patients (Figure1). 72% of young-onset adenocarcinoma diagnoses (n=31) were either moderately or poorly differentiated in nature with 60% of poorly differentiated tumours involving the rectum versus 42% traditional-onset patients (n=17) who exhibited poor differentiation and advanced stage. Rare histology’s were confirmed in both cohorts, with similar levels of Mucinous Cell histology recorded 11%(n=5)/10%(n=4). However, only in young-onset patients was Signet Ring histology present (5%) with 50% of these patients exhibiting metastases.
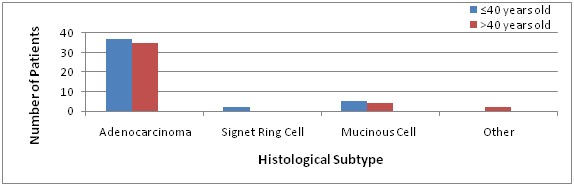
Figure 1 Histological Subtype recorded at Biopsy amongst Young (n=44) and Traditional-onset (n=41) CRC cases.
Presenting symptoms
Young-onset patients most commonly presented with symptoms of abdominal pain 41% (n=18) and rectal bleeding 18% (n=8) (Figure 2). All cases of rectal bleeding and discharge symptoms were found in rectosigmoid cancers with 50% of those experiencing abdominal pain suffering right-sided malignancy. Emergency presentation was more common in young-onset patients (16%/10%) with bowel obstruction the most common presentation, 86% of these occurred in left sided malignancy. Traditional-onset patients (n=39) had varied presentation with Rectal bleeding (n=9)(23%), abdominal pain (n=8) (21%) and change in bowel habit (n=9)(23%) most prevalent. However, while of low incidence, traditional-onset patients exhibited higher incidence of abdominal mass (8%v2%) which in 66% cases was associated with right-sided malignancy and longer duration of symptoms (>6 months). Sole weight loss was an uncommon presenting symptom. However some weight loss was noted in 87% of all patients. Traditional-onset patients were more likely to present with significant weight loss (>10%) (56%/31%).
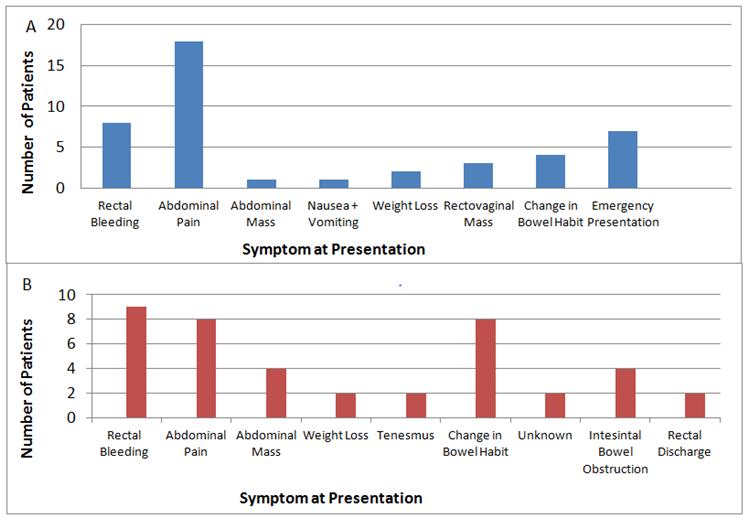
Figure 2 Presenting symptoms in CRC patients designated as (A) Young-onset and (B) Traditional-onset CRC.
Tumour location
Rectal cancer was the most common tumour sub-site in both cohorts although to a greater degree in traditional-onset patients (39%v54%). Young-onset individuals were more predisposed to right-sided tumours (22.73%v17.07%) and were the only cases of transverse colon involvement (5%) and multiple/synchronous tumours (4.55%). Compounding these results in the young was that 82% of patients presented at pathological stage T3/T4 compared to 76% in those >40years, suggesting inherently more invasive disease (Figure 3)
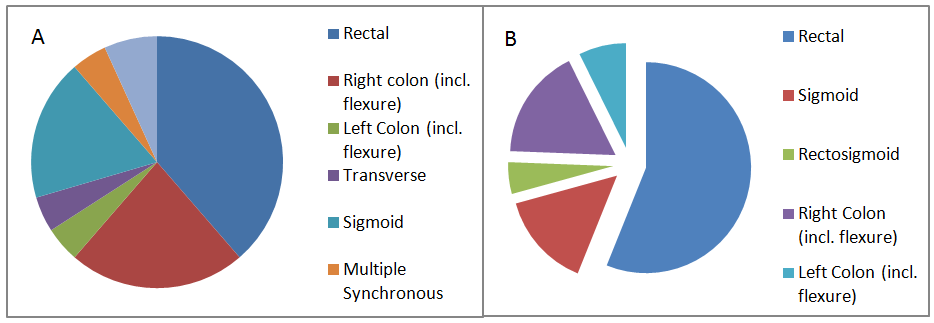
Figure 3 Tumour anatomical sub-site according to age designation (A) ≤40 years old (B) >40 years old.
Baseline CEA levels
Baseline CEA levels (Normal 0-5ng/mL) were measured in 95% (42/44) and 78% (32/41) of young and traditional-onset patients respectively (Figure 4). In both cohorts there was heterogeneity. However this was more present within young-onset patients by virtue of the widened box plot (IQR=12.9 v 6.6). In our young-onset cohort data was negatively skewed with the median of young-onset being recorded as normal (3.08ng/mL). In contrast in our traditional-onset cohort the data was positively skewed with median (2.74ng/mL) and it was in this cohort that non-reactive samples were recorded in histopathologically advanced cases. In both cohorts major outliers existed (518.58 and 874ng/mL).
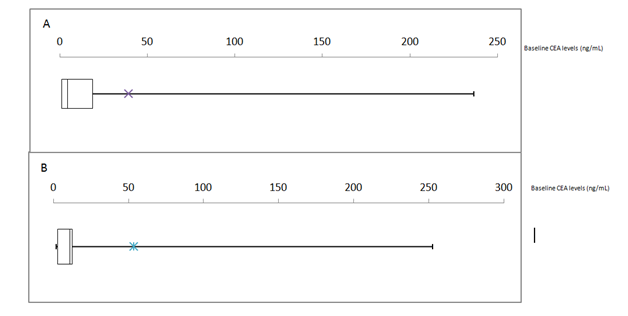
Figure 4 Box-Whisker Plots of Baseline Cancer Embryonic Antigen (CEA) levels in (A) ≤40year old cohort and (B) >40 year old cohort (n=41/44 and 33/41 respectively). *Represents mean CEA values.
Treatment and outcome
87% (74/85) of all patients received standard treatment of which 56.5% (48/85) were managed with curative intent and were equally distributed between young and traditional-onset groups. Young-onset patients were more likely to be enrolled in palliative management programmes (43%/30%) and of these 69% had rectosigmoid cancer and 37% pT4 staging. Of those patients managed curatively 98% (46/48) received at least one surgical intervention, and of these 96% received adjuvant chemotherapy. The most common regimen used was 5-Flurouracil+Calcium Leucovorin (n=19) followed by FOLFOX (n=15) with multiple regimens recorded in ~20% (n=9). Of rectal cancer patients’ ≤40years only 41% (7/17) had completed treatment that included radiotherapy while only 36% traditional-onset patients had radiotherapy as indicated.
At the time of the analysis 2 (2%) had died, 27 were in remission (32%), 20 (24%) were undergoing treatment and 36 had progressive disease (42%) of those ≤40years with progressive disease were more likely to have rectosigmoid cancer (68%), with higher levels young-onset patients (34%) in remission compared to traditional-onset patients (24%). Through long term follow-up of all patients (average 12 months) the incidence of recurrence was recorded. In both groups similar recurrence rates were noted (15%/16%). In young-onset patients with recurrence 5/6(83%) were in Female patients with rare histology, Rectosigmoid/Right-sided malignancy (50%/50%), and emergency presentation 2/6(33%). Outcomes of treatment revealed that patients >40years were most likely to experience complications (n=26) (Table 2). Traditional-onset patients were more likely to suffer post-operative wound infection (15%), urinary retention (19%) and were the only instances of Incisional hernia (12%). In young-onset cases with treatment complications 29% had an oncological emergency of which Neutropenic Sepsis (75%) was most common. In both cohorts low levels of Surgical Revision and Postoperative Nausea and Vomiting
Nature of complication |
≤40years (n=21) (%) |
>40years (n=26)(%) |
Post-op wound infection |
2(10) |
4(15) |
Surgical Revision |
1(5) |
1(4) |
Oncological Emergency |
6(29) |
3(12) |
Fistula |
3(14) |
2(8) |
Bowel Obstruction |
7(33) |
4(15) |
Infection at other Site |
2(10) |
1(4) |
Other |
||
Incisional Hernia |
0(0) |
3(12) |
Anemia |
3(14) |
0(0) |
Urinary Retention/ Incontinence |
4(19) |
6(23) |
Poor Resection Margins |
2(10) |
2(8) |
Post-operative Nausea/Vomiting |
1(5) |
1(4) |
Table 2 Complication of Treatment and Disease Process
Young-onset CRC may represent a clinicopathologically distinct tumour associated with altered outcomes. Its appearance in developing countries has been attributed to westernization of diet and lifestyle, as it has the population structures of said nations which restrict disease in the young.15 Nevertheless, up to 62% of the projected increases in worldwide CRC cases over the next 20years will be in developing nations.16
In keeping with a recent systematic review, young-onset CRC was defined here as ≤40years-old.17 52% of our patients were ’young-onset’ which is far higher than the traditionally quoted 7-10% of western cohorts, and is consistent albeit at a higher proportion than previous reported work from the region.17,18 This difference can be explained by inherent institutional bias and the improved routine use of colonoscopy and advanced medical imaging at this centre. While young-onset CRC has previously been associated with delayed presentation, attributed to superior tolerance of symptoms, low consultation rates and physician misdiagnosis, no appreciable delay was found here with 52% of our young-onset patients presenting within 6 months of symptom onset although as 86% had pT3/pT4 staging perhaps more symptomatic disease and therefore earlier presentation. However, as 23% of young-onset patients presented in the 12-24month range this would suggest patient education of CRC presentation remains an important issue.
Unlike the more varied presentation of traditional-onset patients that included change in bowel habits and significant weight loss, young-onset patients more commonly suffered abdominal pain (41%) and emergency presentation (16%)the latter of which is linked to both poor short and long term outcomes and more invasive disease.4. This preponderance for abdominal pain in young-patients is thought to reflect the effects of ageing on nociceptive afferent fibres and their subsequent desensitisation to neurochemical and mechanical input.19. As such chronic abdominal pain in the context of other CRC symptoms in the young should raise the possibility of CRC even when functional limitations do not exist.
Surprisingly, a large percentage of our young-onset cohort (55%) reported co-morbid disease with HIV present in 15% (Ethiopia HIV prevalence-1.14%).20 HIV has been epidemiologically linked to increased risk of CRC; although Kaposi sarcoma of the Bowel presents similarly.21 As such surveillance of these patients is prudent. In contrast, traditional-onset patients were more likely to have features of the metabolic syndrome. This is important as each 5 unit increase in BMI increases CRC risk by 30% while type 2 Diabetes Mellitus increases risk by 29% and 34% in men and women respectively.22 This indicates the need for improved preventative and management measures for these metabolic conditions in Ethiopia. Identifying at risk young-onset patients is challenging and our data remains inconclusive as to the importance of family history of CRC as this data was unavailable in the majority. Future work should try to relate the age and tumour characteristics of our young-onset CRC patients’ and their relatives to see if the principles of genetic anticipation exist whereby the presence and severity of symptoms presents earlier in successive generations. Good practice should include surveillance of young-family members of CRC patients in this setting with all young-onset cases receiving genetic testing to exclude familial syndromes which currently is often omitted.23
Histopathologically our young-CRC cases had a high incidence of right-sided/rectal tumours, perineural/lymphovascular invasion, nodal involvement, moderate/poor differentiation and metastatic disease which reflects recent reports.11 Mucinous CRC which is favourable in non-metastatic non-rectal disease was reported here at similar levels to western populations (11%). Signet-ring histology however was found more common here than in western cohorts (5%v1%) and was associated with metastasis and poor outcomes which others have reported.24. Both these histology’s and others are linked to underlying microsatellite instability and suggest overlap with Lynch syndrome.25 While the above features are implicated in poor survival, ethnicity is also a determinant of prognosis with black people having the worst prognosis.26
It is important to note however the limitations of this work such as the centres use of multiple private sources for histopathology reporting and tumour biochemistry (CEA). As such standardization of measuring and reporting these results could influence our findings. For instance nodal staging requires ≥12 lymph nodes if it is to avoid understaging which may lead to erroneous use/disuse of neoadjuvant/adjuvant therapy;27 however this practice was underperformed in our cohort (59%/56%). In addition CEA a prognostic tumour marker at baseline and a method for detecting recurrence showed great heterogeneity and asymmetry between our cohorts.28 Indeed 44% (37/85) of our patients had normal baseline levels. This variability and normalization of CEA values may be explained by differential production by anatomical subsite, different methods of its detection, and its sensitivity to benign processes (e.g. smoking). Other limitations include the retrospective analysis of paper health records which were often incomplete or misplaced as well as poor patient educational attainment which may have influenced some of the results reported here. While no statistical analyses were performed we do believe these results have merit in highlighting the different behaviours and outcomes of CRC in Ethiopia by age of onset. The outcome of young-onset CRC remains controversial with studies limited by their small population size. It is thought that despite their predisposition to advanced disease they may still have a better prognosis in low-stage non-metastatic disease.15,29
While determinations regarding survival cannot be made by our data, by the time of analysis two young-onset patients had died and overall a similar rates of recurrence versus traditional-onset patients was recorded (15%/16%) which is concerning and suggests suboptimal treatment. Young-onset patients showed a greater likelihood for palliative management (43%) with 69% of these patients suffering rectosigmoid diseases. This would suggest that rectal disease in this cohort is inherently more hazardous and may be of a different biology and outcome versus other anatomical subsites. Indeed while treatment provision was high overall (87%) only 41%/36% patients of our rectal cancer cohort had appropriate neoadjuvant radiotherapy. Radiotherapy and Total Mesorectal Excision (TME) are considered cornerstones of curative treatment in advanced rectal disease.30 While TME was not performed at this centre access to treatment remains an issue with the current radiotherapy waiting list exceeding one year. Moreover, due to drug unavailability 20% of all patients on chemotherapy experienced cycle-interruptions which can yield resistant subclones and increase long-term recurrence and mortality rates.
However as with any treatment a benefit/harm ratio must be considered. While young-onset patients were less likely to suffer postop-wound infection and surgical complications they did have a high incidence of post-chemotherapeutic febrile neutropenia. This would suggest young-patients are affected greater by current regimes as a result of more invasive underlying disease and higher levels of anaemia and are both risk factors for its development.31 Due to the great risk of mortality with this complication greater surveillance and use of preventative measures in this cohort may be required than in traditional oncological populations.
To conclude in this Ethiopian based study we found high levels of young-onset CRC of which rectal disease was the most prevalent. These patients often had poor histopathological factors such as advanced staging, lymphovascular/perineural involvement and mucinous/signet-ring histology resulting in a high likelihood of palliative management. With this in mind we suggest that young-onset CRC does not behave in the same manner as traditional traditional-onset CRC and showed a trend to inferior prognosis. Much work remains in understanding the pathogenesis of this condition in order to identify potential risk factors and establish new biomarkers that may help identify cases and predict therapeutic responses as there is a high level of treatment complications.
None.
The authors declare that there are no conflicts of interest.
None.

©2017 Worku, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.