Journal of
eISSN: 2373-633X


Review Article Volume 8 Issue 2
1Department of Hematology/Oncology, Kymera Independent Physicians, USA
2Division of Internal Medicine, Department of Hematology/Onclogy, Texas Tech University Health Sciences Center School of Medicine, USA
3Acharya Shri Chander College of Medical Sciences and Hospital, India
4University of Michigan, Ann Arbor, Michigan, USA
5All Saints University School of Medicine, Dominica
Correspondence: Ajaz Bulbul, Kymera Independent Physicians, 101 South Canal St, Carlsbad, NM, USA, Tel 5055048731
Received: June 05, 2017 | Published: July 6, 2017
Citation: Bulbul A, Chouial S, Mustafa A, et al. Feasibility of administering a modified outpatient regimen of vincristine, doxorubicin, cyclophosphamide alternating with ifosfamide, and etoposide (VAC/IE) for high-risk desmoplastic small round blue cell tumor and the role of tumor profiling in selecting treatments. J Cancer Prev Curr Res. 2017;8(2):227-231. DOI: 10.15406/jcpcr.2017.08.00270
The 27-year man presented with slowly progressive abdominal symptoms and multiple large abdominopelvic masses largest measuring 30cm was treated with a neoadjuvant modified VAC/IE regimen commonly employed in Ewing’s sarcoma because of similarities and oncogene activation pathways.1 After 52weeks of treatment, the target lesions shrunk by 80% with this largely modified outpatient protocol followed by radical resection and no evidence of disease. Unfortunately, the patient recurred eight months after his surgery and is currently on antiandrogen Biclutamide and gonadotropin releasing hormone (GnRH) agonist selected based on biomarker evaluation. These biomarkers may have a level of evidence based on study design and validity assigned according to the Literature Level of Evidence Framework consistent with the US Preventive Services Task Force.2
DSRCT originates from a cell with multilineage potential and a molecular hallmark of DSCRT being the EWS-WT1 reciprocal translocation.3,4 Previous work on complex genomic sarcomas has shown novel genetic alterations in sarcomas however DSRCT has been represented in very limited numbers.5
The P6 regimen uses HD-Cytoxan at 4.2gm/m2 dose is a toxic regimen and requires prolonged inpatient stay.6,7 A standard alkylator dose (Cytoxan 1200mg/m2 over 60min) and 1800mg of ifosfamide per square meter per day for five days modified for an outpatient regimen could maintain the quality of life with similar outcomes as seen with more aggressive and toxic regimens.
Genomic and Molecular assessment may help determine the ideal regimen that will help achieve maximal tumor debulking which is crucial to survival.8,9 since large abdominal mass is a usual presentation is aggressive and occurs in adolescents and young adults.8,10
A 28-year-old Hispanic man presented to our cancer clinic after a referral from his primary physician for a newly diagnosed large abdominopelvic mass seen on a CT scan. He had presented to his physician after complaining of up to 6 months of generalized discomfort in his right lower abdomen and constipation which had now progressed in intensity, and he was beginning to notice about 30lb weight loss over the last 3months.
His pain was spasmodic and a self-exam had revealed a hard mass on palpation in the right lower quadrant. He was had occasional night sweats without any objective fever. On his exam, he appeared anxious had no generalized lymphadenopathy and on palpation, a large, poorly defined mass was felt on deep palpation in the right lower abdominal quadrant with mild tenderness. He did not appear cachectic.
Initial contrast CT abdomen and pelvis in August 2014 showed a right lower quadrant, 11.2 x 7.2cm; also central soft tissue mass in the pelvis, 10.7 x 9.5cm, representing a conglomerate lymphadenopathy. No liver or spleen abnormalities were noted. Bilateral moderate hydronephrosis was seen secondary to extrinsic compression of ureters.
A PET scan a month later revealed multiple hypodense lesions in the liver with metabolic activity in segment 6 measuring 4.3 x 1.8. The Spleen demonstrated increased metabolic activity in the superior and inferior poles with SUV up to 19. The central mesenteric mass in was now 12.3 x 8.4cm in size (compared with 10.7 x 9.5cm). Mass in the mesenteric left mid-abdomen SUV of 19 measuring 5.4 x 4.1. Mass in the right lower quadrant is now measuring 14.9 x 12.2 cm in size (compared to 11.2 x 7.2 cm) a month earlier (Figure 1).
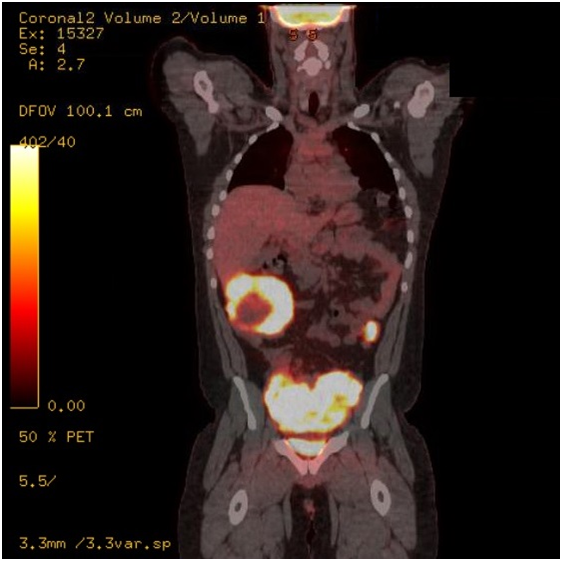
Figure 1 September 2014; Mass in the mesenteric left mid-abdomen SUV of 19 measuring 5.4 x 4.1. Mass in the right lower quadrant is now measuring 14.9 x 12.2 cm in size.
An FNA biopsy of the mesenteric mass revealed insufficient tissue with predominantly reactive and with a fibrotic reaction.
In October 2014 a repeat 18G core biopsy revealed pathological findings consistent with Desmoplastic small round cell tumor with focal rhabdoid features; intense desmoplastic reaction; well-defined islands of cells, surrounded by prominent desmoplastic stroma immunohistochemical reactivity for keratin, epithelial membrane antigen (EMA), neuron-specific enolase (NSE), and desmin as previously described.11,12 RT-PCR was positive for the EWS-WT1 transcript.
Unfortunately given his insurance and logistical issues his treatment could not start until two months later, therefore we decided to have a new baseline scan in December 2014 which revealed the two target size lesions were now 15 x 20 cm with central attenuation in the mesentery with evidence of central necrosis and had reached to just underneath the gallbladder. The pelvic mass is now measuring 30 x 20 cm in the abdominopelvic junction extending into the pelvis (Figure 2). It was decided for him to be started on an alternating VAC/ IE (Vincristine, Adriamycin, Cytoxan/ Ifosfamide etoposide) regimen commonly used in Ewing sarcoma which was starting in November 2015.
In addition to the baseline imaging and an echocardiogram, he received labs at weekly intervals including a complete blood panel and metabolic profile with a UA to detect for occult hematuria. He had to image at regular intervals every 2-3 months. His first follow-up CT revealed three months later in March 2015 showed the mesenteric mass was now 14 x 13 cm and previously 15 x 20 cm; the largest mass in the pelvis now measured 16 x 9.8 x 11.4 (previously 30 x 20 cm) (Figure 3). He had received five cycles of VAC and IE and showed shrinkage of three large mesenteric masses with a significant decrease in size including our target lesions compared to prior study. There was a significant decrease in the size of the subscapular masses in the liver and spleen compatible with response to therapy. After progressive reduction in tumor size his final restaging after 52 weeks in of chemotherapy October 2016 showed the pelvic mass now measuring 6 x 4 x 4 (a year ago, measuring 30 x 20 cm) (Figure 4), an 80% reduction in tumor volume. Both liver and spleen were unremarkable prior to him being operated.
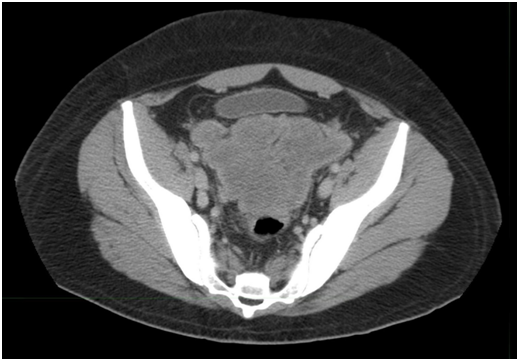
Figure 3 March 2015; after 3 months of VAC/IE. Mesenteric mass was now 14 x 13cm (previously 15 x 20 cm). Pelvic /retro vesical mass16 x 9 cm, (previously 30x20cm).
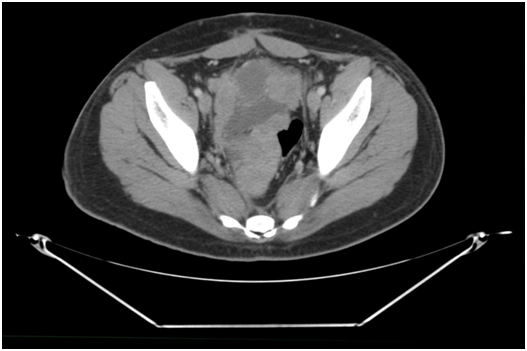
Figure 4 October 2016; 52weeks of chemotherapy showed the pelvic mass now measuring 6 x 4 x 4 (a year ago measuring 30 x 20cm) i.e 80% size reduction.
Next generation tumor sequencing of the resected tumor with MI-X profile (Caris molecular life services) revealed no actionable mutations. Tumor mutational load was low, and PDL-1 was negative. However there was a high ecpression of Androgen (AR) receptors.
DSRCT belongs to the small round blue cell tumor family. The differential includes Ewing sarcoma family tumors including rhabdomyosarcoma, neuroblastoma, lymphoma, small cell carcinoma, neuroendocrine tumors, synovial sarcoma, blastic variant of Wilms’ tumor in children.13,14
A standard Ewing’s sarcoma alternating VAC/IE (Vincristine, Adriamycin, Cytoxan/ Ifosfamide, Etoposide) protocol with more standard alkylator doses (Cytoxan 1200 mg/m2 over 60min) and 1800mg of ifosfamide per square meter per day for five days with mesna.15 was used. The patient received a variation of this due to logistical and insurance issues which meant he couldn't afford to receive his chemotherapy as an inpatient regimen at a tertiary care hospital that was over 100 miles away. Therefore, we devised an outpatient infusion regimen that could be feasible. (Table 1 for chemotherapy protocol) After he had received 400 mg/m2 of Adriamycin dactinomycin was substituted with Dactinomycin 1.25mg/m2.15-17
|
Drug |
Dose |
Administration |
Comments |
|
Vincristine |
2 mg flat dose |
IV in 50mL NS over 15min |
D1 |
|
Adriamycin |
75 mg/m2 |
IVP |
D1 |
|
Mesna |
240 mg/m2 |
IV in 100mL D5W over 15 min |
D1 |
|
Cyclophosphamide |
1.2 gm /m2 |
IV in 500mL D5W-1/2 NS over 1 hour |
D1* |
|
Mesna |
240 mg/m2 |
Hours 4 and 6-8: IV in 100mL D5W over 15 |
D1 |
|
Alternating with Ifosfamide and Etoposide (outpatient schedule) q2-3 weeks. With Growth factor support |
|||
|
Prehydration |
1 liter |
D5 ½ NS1 ltr over 2 hrs |
D1-D5 |
|
Mesna 360 mg/m2 |
360 mg/m2 |
IV in 500mL D5W over 15 min |
D1 (Prior to ifosfamide infusion)-D5 |
|
Ifosfamide |
1.8 gm/m2 |
IV in 500mL D5 ½ NS over 3 hr |
D1-D5 |
|
Mesna |
360 mg/m2 |
IV in 500mL D5W over 15 min |
D1 (Hour 4 and hour 6-8). Hour 4 and 6-8 Mesna could also be given as 720 mg/m2 PO in carbonated beverage |
|
Etoposide |
100 mg/m2 |
Iv in 500 cc NS over 1 hr |
D1 (Given at hour zero) – D5 |
|
D5 ½ NS with 1 amp of Bicarb over 8 hrs (if patient hydrating well and no hematuria IV hydration as stopped after last Mesna dose) |
|||
Table 1 Modified VAC/IE protocol
His treatment over the year was largely uneventful except an episode of gross hematuria that resolved with Mesna infusion and zoster activation soon after he finished the neoadjuvant chemotherapy that was treated with Acyclovir.
He underwent radical resection of pelvic small round cell tumor with en bloc rectosigmoid resection (LAR), extensive ureteral lysis, ureteral stent placement, wide resection of omental mass, resection of descending colon implant, proctosigmoidoscopy, division of right vas deferens for en bloc resection, end-to-end stapled coloproctostomy, partial splenectomy. Operative findings suggested dense desmoplastic tumor involving the bilateral distal ureters, pelvic peritoneum, rectosigmoid colon serosa and right vas deferens, large omental mass consistent with a desmoplastic tumor, desmoplastic nodular tumor in the lower splenic pole and in the descending colon.
Outcome and follow-up
His initial three month postoperative restaging CT scan was negative, however, six months later unfortunately he showed radiological recurrence revealing a large 10 cm left pelvic heterogeneous soft tissue mass with evidence of progression of metastatic disease throughout the abdomen including extensive perihepatic/peri splenic soft tissue implants, interval development of bulky lymphadenopathy in the retroperitoneum and periportal and mesenteric regions. He was clinically asymptomatic with only mild constipation.
Our options for failure in the future are Irinotecan (TEMIRI).18 however IHC for TOPO1 was negative suggested limited benefit from Irinotecan.19,20 and MGMT IHC was positive (60%) suggest a potential lack of benefit,21,22 therefore Gemcitabine docetaxel was used.23-25 given that his tumor was RRM1 negative.26 and TUBB3 negative IHC.27-29 suggesting a potential benefit. The restaging scan however showed progression and the patient received Pazopanib as third line treatment progressing after 2 months. Pazopanib is a small molecule TKI that was approval based on the PALETTE trial.30 DSRCT was not represented in the study. He is currently on antiandrogen Biclutamide and gonadotropin releasing hormone (GnRH) agonist with no futhur clinical deterioration over a month. The feasibility of antiandrogen treatment would need to be studied in a trial setting however with milited toxicit profile it was deemed appropriate.
Aggressive Surgery, radiotherapy, and chemotherapy are the cornerstone treatments of DSRCT. This tumor is somewhat alkylator-sensitive, and response seems dose-responsive.31 Unfortunately, durable responses are limited, and the prognosis for patients remains poor.32 Median survival ranges from 14 to 25years.14 with some reports with patients as young as 5months33 3-year overall survival is dismal at around 44% and the 5-year survival rate a dismal 15%8 with a slightly higher survival reported among those undergoing debulking including 1-2mm implants34 of at least 90% of tumor absence of extraperitoneal metastasis.32 Radiotherapy to high-risk sites, and myeloablative chemotherapy with stem-cell rescue is applied in selected cases especially those with residual disease.31 Hyperthermic intraperitoneal chemotherapy using cisplatin and Yttrium microspheres have been used to treat liver metastasis from DSRCT in limited cases.34
The P6 regimen commonly used for the treatment of DSRCT consists of high-dose cyclophosphamide at 4.2 gm/m2 over two days. The regimen was chosen due to its prior effectiveness and experience of use in and Ewing’s sarcomas.7,16,35 To our knowledge, no data exists in using standard dose modified VAC/IE in an outpatient protocol in this tumor with any success. This regimen affords to maintain the quality of life with largely similar outcomes as seen with more aggressive and toxic regimens. Hotspot analysis has limited scope in sarcoma and techniques such as whole exome sequencing should be explored.5
Learning points
Desmoplastic small round cell tumor (DSRCT) is an aggressive and rare mesenchymal tumor. Its molecular hallmark is the EWS-WT1 fusion protein. The t(11;22) (p13;q12) translocation. It has a predilection of developing in the abdominal and pelvic cavity of young adult men in 88-97% of cases.14,23,36
FDG-PET scanning has been shown to impart important additional information and has a relevant impact on changing treatment planning when used in concert with CT scan.37 Aggressive surgical debulking with the goal to remove >90% of tumor8 is the cornerstone of any curative intent strategy.
Without consolidative therapy with either radiation or myeloablative treatment7-10,36 in most of the studies and relapses occur early without consolidation.
DSRCT is alkylator-sensitive and dose-responsive. Doxorubicin is a common denominator in the treatment of patients who either achieved long-term survival or had responsive tumors.36,38 Evaluating biomarker studies and NGS (Next generation sequencing) may have a limited role to guide us in achieving a targeted and personalized treatment approach in DSRCT.
Low mutational burden suggests these tumors may not respond to immunotherapy. Androgen receptor inhibitors may be feasible combination option in future clinical trials.
None.
The authors declare that there are no conflicts of interest.
None.

©2017 Bulbul, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.