Journal of
eISSN: 2373-633X


Research Article Volume 7 Issue 4
1High Voltage Engineering, Depatment of Electrical and Electronics Engineering, Anna University, Guindy, Chennai, India
2School of Engineering Technology, Purdue University
Correspondence: Raji Sundararajan, 401 N, Grant St. Purdue University, West Lafayette, In 47907
Received: December 16, 2016 | Published: February 28, 2017
Citation: Sree VG, Muthuraman C, Sundararajan R. Electro-cisplatin-therapy for estrogen-receptor positive breast cancers: an in vitro model study. J Cancer Prev Curr Res. 2017;7(4):134-138. DOI: 10.15406/jcpcr.2017.07.00245
There is a critical need for alternate, affordable and physical techniques to treat cancer, especially to treat those inoperable, recurrent, advanced, huge, and bleeding breast cancers that are refractory to current standard of cure. Towards this, electrical pulse-mediated chemotherapy, known as electrochemotherapy is a boon to these patients. This involves application of high intensity, short duration electrical pulses of the order of 1200V/cm, 100µs, which open up pores in the cell plasma membrane enabling increased permeability of usually impermeable or less permeable chemo drug molecules and hence enhanced drug uptake. Due to the increased conductivity and relative permittivity, cancer cells are more susceptible to applied electric field than normal cells. Thus, with the field confined to the tumor, with no or the least effect on normal cells, this technique could be effectively used to treat solid tumors. For this purpose, estrogen receptor positive, MCF-7, human breast cancer cells were studied using the most commonly administered, platinum chemo drug, cisplatin at various doses and their effectiveness is studied using MTT viability assay. With viabilities as low as 30%, our results indicate that we can safely administer cisplatin using electrical pulses. This treatment has the potential to be transferred to the clinical practice.
Cancer is an ancient disease, at least 4000 years old.1,2 This could be due to the fact that it is inherent in our genome and only need certain stimuli, internal or external. Considering that ~80% of cancers are sporadic, it is no doubt it is very difficult to treat cancer. In addition, cancer is not a single disease, but a group of diseases1 and they have multiple pathways to grow, sustain and spread. Thus, it is very difficult to treat cancer, especially, the advanced and metastatic ones. Typically, surgery, radiation and chemotherapy are the three most common modalities of cancer treatments. Surgery and radiation are locally targeted. Chemotherapy involves administering toxic chemicals to either kill or inhibit the growth of cancer cells. It is systemic and usually targets one of the following mechanisms of rapid cell division: a) they damage the DNA, which disrupts DNA replication, b) they interfere with the process of DNA replications and c) they directly interfere with the cell division function.3
Electrical pulses-based chemotherapy is used as an alternative to the current treatment modalities when they are refractory to the patents. Considering that breast cancer is the most common cancer among women, and every 13minutes 6 women are diagnosed with breast cancer and one dies in the USA, we need safe, effective, yet affordable alternate therapies and electrical pulse-mediated chemotherapy, known as Electrochemotehrapy (ECT)4‒12 is a viable alternative. This technique involves application of high intensity, short duration electrical pulses of the order of 1200V/cm, 100µs, which open up pores in the cell plasma membrane enabling enhanced drug delivery. Up to 1000 fold enhancement for a given dose is reported.4 Due to the increased conductivity and relative permittivity, cancer cells are more susceptible to applied electric field than normal cells. Thus, the field is confined to the tumor, with no or the least effect on normal cells. Its success has been attested by the various clinical trials and ongoing treatments for extreme cases of melanomas, sarcomas, and other skin cancers including head and neck cancers.9‒12 When surgery, radio and chemotherapies didn’t work, patients suffering from chest wall breast carcinoma benefitted using ECT.11,12 Thus, electrical pulse-mediated drug delivery is an attractive option to those patients who do not respond to the current standard of cure.
Cisplatin, is a premier platinum compound used commonly for various solid tumors. It causes DNA damage and is one of the first compounds used for treating solid tumors in the 1970s. Since then, it is enjoying its prevalent use in many solid tumors, alone, and in combination with a number of other chemo drugs. Its combination with gemcitabine and veliparib are used for triple negative breast cancer, as first line and second line chemo therapy regimens, respectively. It is also one of the two drugs used in electrochemotherapy. In this study, we used it along with electrical pulses to study MCF-7, estrogen receptor positive human breast cancer cells for its effectiveness in cell kill at various doses.
MCF-7 is a human breast cancer cell line with estrogen, progesterone, and glucocorticoid receptors.13 It was derived from the pleural effusion of a 69year old Caucasian woman with metastatic breast cancer. These are adenocarcinoma cells and are widely used in in vitro studies, because they retain several notable characteristics, especially, mammary epithelium, such as the processing of estrogen via estrogen receptor in the cell cytoplasm.
Figure 1 shows Cisplatin chemical structure,14 with two Cl atoms and two NH3 molecules along with platinum. It was the first chemotherapy drug for treating a solid tumor. It was used along with bleomycin and vinblastine to successfully treat testicular carcinomas, with or without metastasis. Since then it is used extensively for several cancers, including lung, bladder, cervical, ovarian, Head & Neck cancer, esophageal cancer, and triple negative breast cancer, in combination with other drugs, such gemcitabine and veliparib, or alone. While bleomycin is the choice of drug for ECT, cisplatin is also used.4
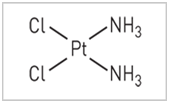
Figure 1 Chemical structure of Cisplatin.14
Platinum compounds produce alkylation of DNA through the formation of reactive intermediates that attack nucleophilic sites. It covalently binds to DNA bases and disrupts DNA function. It binds to the genomic DNA (gDNA) in the cell nucleus and is responsible for its antitumor properties.15 The damage induced upon binding of Cisplatin to gDNA may inhibit transcription or DNA replication mechanisms and trigger cytotoxic processes that lead to Cancer cell death. The toxicity of these agents may be related to DNA damage. Figure 2 shows the mechanism of action of cisplatin.17 Its anticancer action is attributed to the formation of inorganic compounds containing platinum, chlorine and ammonia, which are highly toxic and hence the cell kill via apoptosis as well as the severe nausea and other side effects, such as nephrotoxicity, neurotoxicity, ototoxicity, and vomiting.
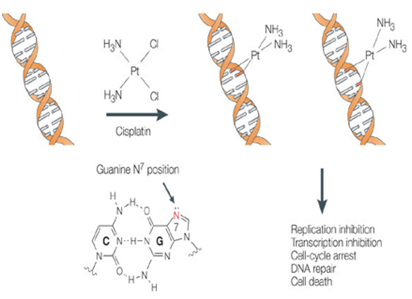
Figure 2 Anticancer activity of Cisplatin by binding to DNA.17
Resistance of platinum compounds could be generalized into four broad mechanistic categories:16 1) decreased alterations in transmembrane cellular drug accumulation; 2) increased cytosolic drug inactivation; 3) enhanced repair of DNA damage, and 4) resistance to apoptosis. It is suggested that coadministration of DNA polymerase alpha inhibitors with cisplatin is useful in overcoming its resistance. Also, it is implicated that platinum sensitivity and resistance are due to alterations in mismatch repair or regulators of apoptosis, such as Bcl-2, Bax, p21, and p53. To overcome cisplatin resistance, modulation of these pathways using therapeutic agents is in development.
Electrical pulses-based chemotherapy is used as an alternative to the current treatment modalities when they are refractory to the patents. Considering that breast cancer is the most common cancer among women, and every 13minutes 6 women are diagnosed with breast cancer and one dies in the USA, we need safe, effective, yet affordable alternate therapies and electrical pulse-mediated chemotherapy, known as Electrochemotehrapy (ECT)4‒12 is a viable alternative. This technique involves application of high intensity, short duration electrical pulses of the order of 1200V/cm, 100µs, which open up pores in the cell plasma membrane enabling enhanced drug delivery. Up to 1000 fold enhancement for a given dose is reported.4 Due to the increased conductivity and relative permittivity, cancer cells are more susceptible to applied electric field than normal cells. Thus, the field is confined to the tumor, with no or the least effect on normal cells. Its success has been attested by the various clinical trials and ongoing treatments for extreme cases of melanomas, sarcomas, and other skin cancers including head and neck cancers.9‒12 When surgery, radio and chemotherapies didn’t work, patients suffering from chest wall breast carcinoma benefitted using ECT.11,12 Thus, electrical pulse-mediated drug delivery is an attractive option to those patients who do not respond to the current standard of cure.
Cells
MCF-7 cells, obtained from National Centre for Cell Sciences, Pune (NCCS), India were used. The cells were maintained and cultured as indicated in the guidelines of ATCC. The reagents used to impart radioactive substrate to the cells are purchased from Cistron laboratories (Secunderabad, India). Trypsin, methylthiazolyl diphenyl- tetrazolium bromide (MTT), and Dimethyl sulfoxide (DMSO) are purchased from Sisco research laboratory chemicals, Mumbai. All other chemicals and reagents were obtained from Sigma Aldrich (Mumbai, India).
Chemotherapeutic drug
Cisplatin, originally from cis-platinum (sp-4-2)-diamminedichloridoplatinum), is a chemotherapy drug approved by the Food and Drug Administration (FDA) in the 1970s.
Electrical pulse generator
A 0.7 to 2kV pulse generator was designed and developed for this study. Figure 3 shows the generator built along with the output waveform generated. It generates unipolar, rectangular pulses, whose magnitude can be varied.
Electrodes
A two needle electrode array was used for electroporating the cell samples.
Test protocol
Three types of samples were tested.
MTT viability assay
The viability of the various samples of MCF-7 cells was determined using MTT assay. Cells (1×105/well) were plated in 1mL of medium/well in 24-well plates (Costar Corning, Rochester, NY). After 48hour incubation, once the cell reaches the confluence, the cells were treated with various drug doses with and without pulses, and the samples were incubated in 0.1% DMSO for 48h at 37°C. After removal of the sample solution and washing with phosphate-buffered saline (pH 7.4), 200µL/well (5mg/mL) of 0.5% 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide cells (MTT) phosphate- buffered saline solution was added. After 4h incubation, 0.04M HCl/ isopropanol are added. Viable cells were determined by the absorbance at 570nm. Measurements were performed and the concentration required for a 50% inhibition of viability (IC50) was determined graphically. The absorbance at 570nm was measured with a UV-Spectrophotometer using wells without sample containing cells as blanks. The effect of the samples on the proliferation of MCF-7 was given in terms of Percentage-specific Cytotoxicity (%C) and is calculated as in literature.18
Effect of cisplatin only on cell viability
Figure 4 shows the dose curve-the viability at various doses of cisplatin. It can be seen that with the increase of the dosage, the cell death increases, which is expected. However, the difference is not linear. For example, by increasing the drug dosage from 125µg/mL to 500µg/mL, a 400% time increase, the viability changed only 32.2%, indicating the complex phenomena of drug reaction. Similar results were also obtained by other researchers for MCF-7 cells (Figure 5),19 where with increase in molarity, the cell viability decreased. Figure 6 shows the microscopic pictures at 0 (control), 62.5 and 125µg/mL concentrations. With 0µg/mL (with no treatment), there are a number of viable cells. With increased dosage, there are more cells killed as seen by the spare spread of the cells.

Figure 5 Viability of the MCF-7 cells at various dosages of Cisplatin in a previous study.19
Effect of pulse only on cell viability
Figure 7 shows viability at two voltage stresses, of 1080V and 1280V and the control. Control (condition 1) is at 100% viability, and the 1080V has a viability of 69%, while it is 58.1% for 1280V. In this case, the variation is almost linear. For a change of 18.52% (from 1080V to 1280V), the viability varied 15.8% (from 69 to 58.1). This illustrates the linear impact of applied voltage on the cell viability, while all other conditions are the same. Figure 8 shows the corresponding micrographs, with control having a more number of viable cells compared to the other two samples with applied voltages.
Effect of electrical pulses+cisplatin on viability
With electrical pulses and the chemo drug, the synergistic effect of the combination could be observed. Figure 9 shows the viability of MCF-7 cells at various drug dosages and applied voltages. Considering the control at 100% (data not shown), the viabilities at 250, 125 and 62.5µg/mL are 38.1%, 45.4% and 50.9% for the applied voltage of 1080V. At 1280V, they vary as, 30.9%, 40%, and 47.2% for the same three concentrations of 250, 125 and 62.5µg/mL. By decreasing the drug dosage 50% from 250 to 125µg/mL, at 1080V, the viability increased 19%, and it was 29.45% at 1080V. Similar, nonlinear variations were observed at other concentration changes. Comparing the viability changes at the two different voltages, for a given concentration of 125µg/mL, the viability change was 11.89%, compared to the voltage change of 18.5%. This nonlinear viability variation indicates the complexity involved in the cell kill due to the application of electrical pulses and administration of chemo therapeutic drugs, and their synergy. In general, there is more cell death due to combination of pulses and chemo drug, than either pulse only or drug only at the applied voltages and administered dosages.
Figure 10 shows the micrographs, at the two voltages and at two dosages of 125 and 62.5µg/mL, along with control, again illustrating less number of cells (less viability) compared to the control, when pulses and cisplatin were used. Table 1 shows a comparison of cisplatin only vs cisplatin+electrical pulses, illustrating the enhanced efficacy obtained with electrical pulses, so, a lower dose could be used effectively.
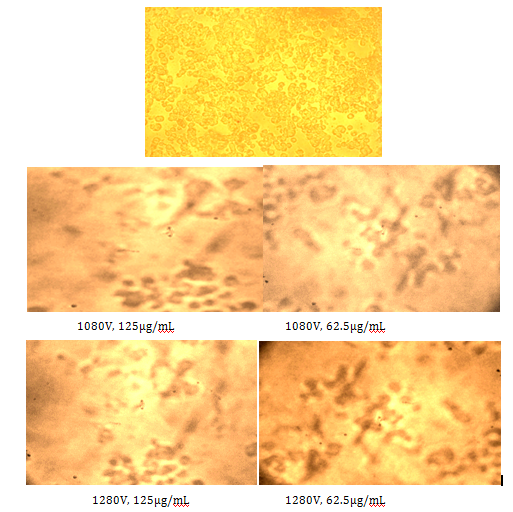
Figure 10 Micrographs of the cells at various voltages and drug concentrations: Control, 1080V, and 1280V (from left to right).
Dosage, µg/mL |
% Viability |
||
Cisplatin only |
Cisplatin+1080V |
Cisplatin+1280V |
|
125 |
50.9 |
45.4 |
40 |
62.5 |
61.8 |
50.9 |
47.2 |
Table 1 Comparison of Cisplatin only vs Cispltin+Electrical pulses
The properties of MCF-7 human breast carcinoma cells make them resistant to cisplatin, a potent anticancer drug, compared to other breast cancer cells. In order to enhance the efficacy of cisplatin in treating MCF-7 breast cancer cells, it was uploaded using electrical pulses, which open-up aqueous pores that allow the uptake of the drug into the usually impermeant cell membrane. With drug only, the viabilities varied from 34.5% to 74.5% at drug doses varied from 500 to 31.2µg/mL. When tested with PEF only, the viabilities varied from 69% to 58% for voltages from 1080V to 1280V. However, when drug and PEF were used, the viability reduced to 45.4% for a concentration of 125µg/mL and 50.4% for 62.5µg/mL. Considering the low dose of the drug, it shows the potential of the combination therapy for clinical applications. This is, especially useful for chemo resistant breast tumors. This technique could be widely used to treat those tumors that are refractory to current standard of treatment. This treatment has the potential to be transferred to the clinical applications.
The authors are extremely grateful to Life Tech research centre, Chennai for their help with acquiring cell lines during the course of the experiments.
Author declares there are no conflicts of interest.

©2017 Sree, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.