Journal of
eISSN: 2373-633X


Case Report Volume 6 Issue 3
Department of Radiation Oncology, Ram?n y Cajal University Hospital, Spain
Correspondence: Fernando Lopez Campos, Radiation Oncology Department, Ramon y Cajal University Hospital, Ctra. Colmenar Viejo Km. 9,100, 28034, Madrid, Spain, Tel 34 663158959
Received: September 30, 2016 | Published: December 9, 2016
Citation: Candini D, López-Campos F. Small cell prostate cancer: a potential prophylactic treatment for a rare and aggressive tumor. J Cancer Prev Curr Res. 2016;6(3):462-465. DOI: 10.15406/jcpcr.2016.06.00205
Small cell prostate cancer (SCPC) is a rare pathology, with aggressive behaviour and a high tendency to metastasize. We present a case report of a 62-year-old male who underwent prostatectomy due to a rising prostate specific antigen (PSA) value and a positive biopsy for prostate cancer. Pathology results revealed a poorly differentiated adenocarcinoma of the prostate gland, Gleason score of 4+5=9, with extensive areas of undifferentiated small cell carcinoma. Despite aggressive adjuvant chemotherapy, hormone deprivation treatment and radiotherapy, the patient developed a castration-resistant prostate cancer with oligometastatic disease, diagnosed by choline positron emission tomography - computerized tomography (PET-CT). We discuss the clinical aspects correlated with this unusual subgroup of prostate cancer, adding a literature review to talk over potential prophylactic treatments in order to prevent central nervous system lesions, or potential discontinuation of hormone deprivation treatment if poor response is achieved.
Keywords: small cell prostate carcinoma, prophylactic cranial irradiation, choline pet-ct, oligometastatic disease
PSA, prostate specific antigen; PET-CT, positron emission tomography-computerized tomography; SCPC, small cell prostate cancer; PCI, prophylactic cranial irradiation; PC, prostate cancer; SCLC, small cell lung cancer; WHO, world health organization; CRPC, castration-resistant prostate cancer; ESCC, extrapulmonary small cell cancer; OS, overall survival; NNT, needed number to treat
The incidence of prostate cancer (PC) has increased worldwide, allegedly since the accepted use of PSA as a screening tool. Even though most PC are adenocarcinomas, other subtypes have been defined, namely squamous cell, sarcomatoid, urothelial, basal cell, adenoid cystic and small cell carcinoma. Each one of them has specific clinic-pathologic features, distinct clinical relevance and different prognosis.1 The diagnosis of small cell prostate cancer (SCPC) is reached based on the presence of morphological features similar to those found in SCLC, as defined in the 1999 WHO classification criteria of pulmonary neoplasms (Figure 1): proliferation of small cells with unique morphological features, little cytoplasm, poorly-defined borders, finely granular ‘salt and pepper’ chromatin, absent or inconspicuous nucleoli, frequent nuclear molding and a high mitotic rate.2
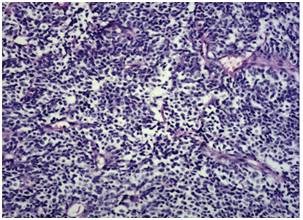
Figure 1 Histological findings of the prostatic tumor. H&E (x20).2
SCPC is an aggressive, rare tumor representing less than 1% of all prostate cancers, although autopsy studies have suggested it may be present in up to 20% of castration-resistant disease.3 Its appearance can be accompanied by normal PSA values and rapid tumor growth including extensive local and visceral involvement, clinically evident in advanced disease that confers a grave prognosis.4 Main symptoms are signs of urinary obstruction and of metastatic disease, like bone pain, neurologic manifestations, hydronephrosis or abdominal pain. The bones, central nervous system, liver, regional and distant lymph nodes are the most common sites of metastasis. The treatment algorithm of SCPC is still highly controversial in oncology due to its aggressive nature, uncommon features and poor response to androgen deprivation therapy.1
The first documented case of SCPC was described by Wenk et al.,5 Since then, many other case reports have been recorded.6‒14 However, the majority of these cases have been reported in case series, limiting definitive decision making regarding the most effective treatment of this disease. Overall survival in this subtype of PC has not changed significantly over the last decades, in contrast to the improvement in survival noted for limited stage SCLC, where chemo-radiation is the standard of care in many cases. The optimal treatment strategy for SCPC has not yet been defined, but worldwide accepted treatment modalities are a combination of surgery, radiation and chemotherapy.12
An asymptomatic, caucasian and previously healthy 62year-old man began urologic study on March 2011 after the finding of an elevated PSA value, which raised up to 5.77ng/mL. A rectal digital prostatic examination revealed an enlarged irregular prostate, with a palpable right-side nodule. A transrectal ultrasound-guided prostate biopsy was performed and the pathologic examination revealed a Gleason score of 4+3=7 prostatic adenocarcinoma, affecting both lobes. A chest-abdomen-pelvis CT examination, a head CT scan and a bone scan were performed and resulted in stage T2bN0M0 disease, with no evidence of distant disease.
Treatment alternatives were discussed with the patient, who preferred the surgical option. A laparoscopic radical prostatectomy and bilateral lymphadenectomy were performed, with a definitive histopathological result of poorly differentiated adenocarcinoma of the prostate with extensive areas of undifferentiated small cell carcinoma, Gleason score of 4+5=9. The tumor had extracapsular extension, perineural infiltration and an infiltrated apex, bladder neck and seminal vesicles; 13 lymph nodes were isolated without tumor infiltration. Taking into account the histological diagnosis, definitive TNM tumor stage of pT4 (+) N0M0 and a post-operative PSA value of 0.590ng/mL, the patient was treated with a combination chemotherapy of cisplatin and etoposide for six cycles.
After the sixth cycle, no biochemical response was observed with a PSA value of 1.801ng/mL on March 2012. Post-operative radiation treatment to the bed prostatectomy reaching a total dose of 72Gy with a conventional fractionation of 2 Gy/day was performed; simultaneously hormone deprivation treatment with LHRH analogue was started. No prophylactic cranial irradiation was administered. No recurrence was observed during follow-up, reaching a nadir PSA value of 0.001ng/mL on January 2014, twenty months after radiation treatment. The patient had been free of recurrence until April 2016, when PSA value raised up to 2.363ng/mL with a testosterone value of 18.5ng/mL, fulfilling criteria of biochemical recurrence and castration-resistant prostate cancer (CRPC).15 At all times during follow-up, no evidence of disease was noticed in CT scans or bone scans. In August 2016, after reaching a PSA value of 5.098ng/mL, we requested a choline-PET/CT which showed pathological uptake in a right external iliac chain lymph node and a focal suspicious deposit in occipital bone.
SCPC is a very aggressive disease; reported incidence of metastasis at diagnosis varies from 33% to 75% in the literature. Deorah et al.,12 analysed 191 patients collected from the Surveillance, Epidemiology and end Results (SEER) database of the National Cancer Institute over a 30-year period (from 1973 to 2003), and metastatic disease was the initial presentation in 60% of the patients with a 2-year survival rate of 27% and a 5-year survival rate of 14% (Table 1). SCPC most commonly involves bone, liver, central nervous system and lung; very often it can present with a variety of symptoms, mostly lower urinary tract signs or neurologic findings, and in a minority with constitutional symptoms, haematuria, and hematospermia or paraneoplastic syndromes. In contrast to adenocarcinoma, bone lesions tend to be lytic with associated bone pain and pathologic fractures.3
|
Authors |
Study size (n) |
Metastatic disease (%) |
Cerebral metastases (%) |
2-year overall survival rate (%) |
Median survival (months) |
|
Deorah et al.,12 |
191 |
60,5 |
28 |
27,5 |
21 |
|
Stein et al.,14 |
30 |
42 |
0 |
--- |
17 |
|
Spiess et al.,10 |
83 |
75 |
… |
13 |
13 |
|
Asmis et al.,11 |
10 |
80 |
20 |
--- |
9,5 |
|
Rubenstein et al.,13 |
7 |
71 |
… |
--- |
10 |
|
Oesterling et al.,9 |
27 |
--- |
… |
--- |
17,1 |
|
Tetu et al.,7 |
20 |
75 |
… |
--- |
18 |
Table 1 Comparison between case series of SCPC
The onset of SCPC is younger (usually between the ages of 40 and 60years) than in adenocarcinoma.4 In contrast to prostate adenocarcinoma, serum levels of PSA do not correlate with disease activity and are not useful for post-treatment surveillance or as a surrogate marker of treatment benefit in the setting of advanced disease. Wang et al.,8 described that PSA values ranged from undetectable to 1896ng/mL, with a median value of 4ng/mL. Elevated serum PSA levels could only be detected in patients with SCPC with a substantial amount of adenocarcinoma component; this explains the raise of PSA value in our patient. As with other small cell cancers, SCPC is thought to arise from either neuroendocrine cells that are derived from amine precursor uptake decarboxylation (APUD) cells or totipotent stem cells present in the prostate (1). Primary SCPC is known to arise in a pure form or mixed with prostate adenocarcinoma, as in the case of our patient. Pure SCPC accounts for 36-67% of all cases of SCPC.14 Wang et al.,8 described the pathological features of SCPC, reporting that the Gleason score of mixed adenocarcinoma ranged from 4 to 10, with 85% of these cases being graded as ≥8, suggesting that SCPC appears to be at one end of the spectrum of prostate adenocarcinoma differentiation, representing the worst grade. In mixed tumors, the Gleason grade is applied only to the non-small cell component.
The neuroendocrine differentiation of small cell carcinoma bears poor response to androgen deprivation therapy because of the presence of chromogranin A, a neuroendocrine marker that might be elevated if there is a neuroendocrine differentiation of the carcinoma, and that is known to activate androgen receptors even in the absence of androgens.16 Also many others immunohistochemical markers have been detected in small cell carcinomas, including synaptophysin, ACTH, serotonin, HCG, calcitonin, TSH, and inhibin. A small proportion of these tumors do not stain for any neuroendocrine markers.17 Thus, with increased life expectancy resulting from more-effective targeted therapies of the androgen receptor signalling axis, the incidence of prostatic SCPC could increase as a result of a clonal pressure applied on malignant prostate cells. Therefore, early recognition of histological or clinical features of prostatic SCPC might influence the duration, the discontinuation and the timing of hormone deprivation treatment.
Undoubtedly, our patient could belong to a specific subgroup of metastatic prostate cancer called oligometastatic prostate cancer patient. This term “oligometastatic” was introduced for the first time by Weischelbaum & Hellman18 and it describes a specific situation where a patient has distant disease in a limited number of regions (less than five) and where the primary lesion can be controlled. Modern techniques such as positron emission tomography (PET-CT) have a major sensibility in terms of metastasis detection compared with CT or bone scan, a key for classify this kind of patients.19 Different radiolabels, that are useful for prostate cancer diagnosis and tracking, are in development, including markers of cell membrane proliferation (11C/18F-choline). Nowadays the most widespread technique for receive stereoctatic body radiation treatment (SBRT) of oligometastatic prostate cancer is PET-CT Choline.20 In our case, the patient is going to receive a SBRT treatment at two targets, an iliac lymphnode and the occipital bone.
Given its rare presentation and the lack of randomized and multicentre studies, there are no specific guidelines for the treatment of SCPC. For localized disease, radical prostatectomy with lymphadenectomy or high-dose radiation treatment including pelvic lymph nodes radiation is worldwide suggested, although multimodal treatment with the addition of systemic treatments, like cisplatin and etoposide or other agents are often recommended. Spiess et al.,10 justifies this treatment intensification to maximize the potential survival benefit with acceptable and tolerable adverse effects. Recently, Bex et al.,21 showed promising results for non-metastatic small cell cancer of the bladder (SCBC), where 17 patients with SCBC underwent transurethral resection of their tumor and were then treated with platinum-based chemotherapy followed by radiotherapy. A complete local response was achieved in 15 patients (88%) with a median overall survival of 33months. Whether or not this approach can be applied to localized SCPC remains to be seen.
These theories have been extrapolated from the treatment of SCLC, in which prophylactic cranial irradiation (PCI) had been recommended for patients who have achieved complete or partial response after a chemo-radiotherapy treatment. There is a lack of consensus on whether patients with extrapulmonary small cell carcinoma (ESCC), like SCPC, should be treated with PCI.22 For small cell cancer of the bladder, cervix of oesophagus PCI is not routinely recommended because there appears to be a lower incidence of brain metastases in those ESCC compared with SCLC.23 Underlining that brain metastases, which are extremely rare in prostate adenocarcinoma (0.05% to 0.5%), are much more common with SCPC (16-19%), an interesting approach could be to adapt the treatment paradigm of SCLC and evaluate chemo-radiation as a treatment option for loco-regional disease followed by prophylactic cranial irradiation (PCI) in patients with radiologic responses.24
Eckert et al.,25 reported 51 patients treated from 1999-2011 for ESCC, 11 presented cerebral metastases. The authors calculated the needed number to treat (NNT) based on the relative risk reduction of 60% observed in the studies of PCI for SCLC, resulting in an NNT of 13. These results might be a reason to discuss and evaluate PCI for SCPC patients responding to initial therapies and to set brain imaging as part of staging and restaging in this disease, especially in high T stage or symptoms or signs suggestive of central nervous system disease.26,27 Although PCI can be associated with both acute and long-term neurologic toxicity, research efforts to minimize the neurotoxicity of PCI have been incorporated, including more feasible fractionations (25Gy in 10 fractions or twice daily fractionation of 1.5Gy up to 30Gy), hippocampal-sparing whole brain radiotherapy and the use of alternative concomitant systemic agents.28
Two randomized trials for patients with SCLC in complete remission evaluated PCI neurotoxicity as primary endpoint.29,30 In the first study,29 cognitive function and quality of life were both determined before and after the treatment with PCI or control group (no PCI): neither study demonstrated adverse effects attributable to PCI. In the other trial,30 patients were randomized to receive PCI or to no receive PCI and went through neuropsychological questionnaire and brain CT scans, which were repeated during the follow-up: no significant differences were observed in neuropsychological function. There are no prospective data to inform the role of PCI for patients with SCPC, and in many institutions, it is not performed, but our impression is that even so limited information suggests that PCI may play a crucial role in the management of SCPC. Further data are needed to support this theory in the setting of patients with SCPC and partial or complete response to local treatments.
In summary, SCPC is a rare and aggressive prostate cancer variant that is biologically, clinically and morphologically distinct from the adenocarcinoma subtype. Further studies are needed in order to clarify the roll of PCI and the optimal treatment of SCPC.
None.
Authors declare there are no conflicts of interest.

©2016 Candini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.