Journal of
eISSN: 2373-633X


Research Article Volume 6 Issue 1
Imeddo Inc, USA
Correspondence: Aron B Goins MS DSM, iMedDo Inc, Friendswood, TX, USA
Received: September 21, 2016 | Published: November 9, 2016
Citation: Goins AB. Spectroscopic assessment of magnascent® iodine. J Cancer Prev Curr Res. 2016;6(1):402-405. DOI: 10.15406/jcpcr.2016.06.00191
Patented magnetically treated iodine, Magnascent® Iodine, health supplement iodine tincture (MAG), is analyzed using absorbance spectroscopy and is compared to raw, unprocessed iodine tincture (RAW) of identical concentration. Analysis is performed using both standard (2nm bandwidth) and hi-resolution (0.1nm bandwidth) spectroscopy for both undiluted and diluted samples using both spectroscopic grade ethanol (SpecA) and distilled water (DW). MAG is observed to have a lesser absorbance at peaks near (351nm & 288nm) when diluted in DW and more noticeably lesser absorbance at peaks near (359nm & 291nm) when diluted in SpecA. Spectra for MAG & RAW obtained using high resolution spectroscopy are obtained and subtracted (MAG - RAW) to better visualize differences. No difference is seen in the infra-red (IR) region (701-900nm), but negative difference (lower MAG absorbance) is seen in the visible (400700nm) spectra at ~500-645 nm and negative difference is seen in the ultraviolet (UV) region (190 - 399nm) at the two major peaks for samples diluted in water or alcohol (DW: 248.3nm, 323.3nm), (SpecA: 259.1 nm, 324.8 nm) and the minor peak/quasi peak (DW 205nm), (SpecA ~225nm). This work clearly demonstrates differences in absorbance of nascent and raw iodine. Decreased absorbance of Magnascent® Iodine supports the hypothesis that that the diatomic iodine has been homolytically cleaved into monoatomic iodine because of the electromagnetic process.
Keywords: magnascent, nascent, iodine, spectroscopy, monatomic iodine, diatomic iodine, testimonials, electromagnetic
Iodine
Iodine is a truly fascinating element which seems to defy ordinary chemical classification. Here is some of what Sir Davy Humphry had to say about it circa 1814 when examining the violet gas and dark crystals upon their recent discovery, "In its specific gravity, lustre, the high number in which it enters into combination and colour, it resembles the metals; but in all its chemical agencies it is more analogous to oxygen and chlorine ...".1 The word iodine refers to the element iodine which has 53 protons which can exist in many forms such as monoatomic nascent i.e. atomic iodine (53 electrons), monoatomic iodine (54 electrons) or diatomic molecular iodine I2. Typically the word iodine refers to the diatomic violet gas I2(g) or the black/purple metallic diatomic solid crystals I2(s) However, for the purposes of nutritional supplementation, iodine refers to "liquid iodine" which refers to diatomic solid (and/or monoatomic iodide) dissolved in a liquid solvent which if alcohol is referred to as an "iodine tincture".2 Liquid iodine is highly prized as a health supplement as dietary deficiency in iodine is rampant and leads to a variety of deficiency disorders.3 A useful property of liquid iodine is that it has a dark varying color (typically yellow/red/brown depending on solute & solvent concentration and identity), making it particularly amenable to absorbance spectroscopic analysis.
Nascent iodine historical to present
Approximately a century after the discovery of iodine, "nascent iodine" is proposed to be useful for treating nose inflammation (lupus nasi) and tuberculosis.4 However, since the term nascent simply means newborn/freshly created, there is some ambiguity as nascent iodine referred to in 1913 is likely referring to nascent silver iodide (freshly precipitated AgI from reacting KI with HNO3) which was used at the time in Pharmacy.5 The disambiguated term "nascent iodine" refers to the idea of the nascent state developed for hydrogen and oxygen (dating back to ~1806) as applied to iodine (dating back to ~1814), and still being developed by chemists during World War II (1942) essentially refers to the overarching idea that through electromagnetic stimulation of a species a more reactive species can be formed. As evidence and understanding of the nascent state continued to develop in chemistry,6 the modern understanding of nascent iodine in liquid iodine chemistry7 evolved. In modern terms, nascent iodine refers not only to iodine solutions activated by electromagnetic stimulation, but can even more specifically refer to reactive atoms of nascent atomic iodine (zero oxidation state, 53 electrons one of which is as free/unpaired electron, 7 of which are in outer electron shell lacking an octet) and this bizarre atom has a number of different abbreviations (I°; I*; I•; ½I2). Nascent iodine can be created using a variety of ways, including chemically by oxidizing iodide in the presence of acid and iodate8,9 as well as a variety of spectroscopic ways such as released using photolysis from methyl~iodide using 193 nm excitation.10
Magnascent Iodine, nascent iodine produced using a patented electromagnetic process,11 has enjoyed widespread usage by missionaries for not only viral diseases such as dengue fever and chikungunya fever, but has even been used successfully for malaria.12 Although iodine prepared through electromagnetic stimulation is in fact nascent in the overarching sense of having increased health activity (testimonials) after electrical stimulation, such liquid iodine solutions have complicated iodine chemistry, which requires further scientific evaluation to figure how much of different iodine speciation is present including nascent iodine in the modern sense. Quantum mechanical models of iodine developed using spectroscopic absorbance of the gas phase indicate that nascent iodine can be made two ways from either an unexcited or an excited electronic state of diatomic iodine,13 but these models do not apply in liquid iodine solutions. However, iodine chemistry gets overly complicated very quickly in the liquid phase tincture,7 as additional forms can be present such as tri~atomic iodines I3", and a variety of other species such as periodates (anionic salts of iodine and oxygen) can exist in varying forms with varying oxidation states (I+; I3+; I5+; I7+), and perhaps even unknown species as well. All the complexities of iodine solution chemistry notwithstanding, the purpose of this work is to gain an overall insight into the iodine solution chemistry of Magnascent® iodine health supplement using spectroscopic observation by acquisition and comparison of spectral signature to that of unprocessed liquid iodine. The hypothesis tested is that nascent iodine will have a decreased absorbance in some regions of the spectra likely on account of homolytically cleaved diatomic iodine having been pulled apart by the electromagnetic process.
Absorbance spectra basics
Description of absorbance spectroscopy basics can be found in any introductory analytical chemistry book.14 Samples absorb light, and if the wavelength (related to energy) of the light is varied and the absorbance plotted, then this is called a spectrum (spectra plural). A machine capable of measuring spectra is a spectrophotometer, and the Beer~Lambert law applies (A=e*l*C) where absorbance (A) is linearly proportional to three parameters (e) the molar absorptivity, l (pathlength), and C, the sample concentration. Absorbance is found experimentally using a known relationship related to the ratio of light (P/Po) transmitted (transmittance T where T=P/Po) through a sample (A=log(1/T)=log(Po/P)). In this study, l and C are held constant when comparing processed vs. unprocessed iodine, and thus differences in absorption if seen are most likely differences in e (or small unintended differences in C); however, detailed discussion of e is beyond the scope of this work. Instead, we look a general changes in absorbance to look for readily observable differences in different parts of the spectra. For the purposes of this work, spectra is from 190-900 nm, and consists of three regions, the ultra~violet (UV) region which consists of high energy wavelengths <400nm (190 to 400nm), the visible (VIS) region which consists of wavelengths visible to human eye (400-700 nm), and the lower energy infra~red (IR) region >700nm (700-900nm).
Iodine materials
Patented, processed, electromagnetically treated Magnascent® Nascent Iodine 2% Health Supplement (MAG) is obtained from Magnascent Division of Shield Bearer Inc, Azle, Tx, USA. MAG is created from 440,000 mcg iodine total per 1 ounce bottle. A sample of the pre-processed iodine from the same batch was provided for comparison and is of identical total iodine concentration (440,000 mcg iodine total per 1 ounce bottle) known as raw iodine tincture (RAW), which is unprocessed, un-electromagnetically treated iodine in alcohol. Alcohol solvents used are 192 proof (96% pure) grain ethanol. Iodine used to create tinctures refers to diatomic I2 solid state iodine crystals obtained by Shield Bearer from an undisclosed third party company with Shield Bearer disclosure that "crystals from resublimated US mining source," and "not from the ocean (not contaminated by Fukushima reactor leakage) or from animal processing (Vegan friendly)," and "no heavy metals detected." Author verified the above statements through 3rd party heavy metals testing (see bottom of www.magnascent.com), and by contacting 3rd party iodine supplier after signing a non-disclosure agreement.
Spectroscopy
Spectroscopy is performed with a Jasco Inc. (Tokyo, Japan) V-650 Spectrophotometer. Standard resolution spectra are obtained using the following parameters and settings: bandwidth 2.0nm, response fast, 900-190nm spectra, scan speed 2000nm/min, no correction, data interval 1.0nm, 10 accumulations, standard 10mm cell holder attachment using a 1mm quartz cuvette. Spectra of MAG & RAW are obtained in undiluted solution, analyzed diluted in spectroscopic grade alcohol (Sigma-Aldrich, St. Louis, MO, USA) denoted (SpecA) for convenience, and analyzed diluted in distilled water (DW). Dilutions are performed by putting 50μL of sample (MAG or RAW) into 450μL of solvent into ~500μL 1mm pathlength quartz cuvette. Additionally, high resolution spectra are obtained with parameters as described above but with bandwidth 0.1nm, speed 100nm/min, baseline correction, data interval 0.05nm, no accumulation for MAG & RAW undiluted, and with DW dilution (50μL into 450μL) & SpecA dilution (10μL into 490μL). Hi-Resolution data (0.1nm bandwidth) is summarized by subtracting the RAW from the MAG spectra signal for each solvent using JASCO's Spectra Analysis Algorithm Subtraction function so that differences can be readily visualized. The MAG-RAW spectral difference plot examined over 190 to 900nm is expected to be zero for wavelengths where no absorbance difference is seen (RAW=MAG so MAG-RAW=0), positive where RAW>MAG and negative for wavelengths where the absorbance of MAG<RAW.
Spectra of magnascent vs. raw iodine
Unprocessed iodine, RAW, and patented processed nascent iodine, MAG, are both very dark (very high absorbance) liquids appearing dark brown upon visual inspection. If absorbance is too high (Absorbance=4 is the maximum linear range of the V-650 spectrophotometer), then either the path length, 1, or the concentration, C, of the sample must be reduced. As path length has already been reduced as much as possible (use of a lmm rather than standard 10mm cuvette), then sample dilution may be required.
Also note that a quartz (glass) rather than a plastic cuvette is required to see spectra in the UV. Standard resolution spectra (2nm bandwidth) of MAG & RAW are obtained in undiluted solution, analyzed diluted in SpecA, and analyzed diluted in DW (Figure 1). As expected, the undiluted samples have very high absorbance (>4) in both the UV and much of the VIS region. Figure 1 shows that no obvious difference between RAW (dark green) & MAG (dark blue) is seen for the undiluted samples as they overlay very similarly (only dark blue upper layer readily visible in the graph). Although not expected to be linear with concentration, many interesting peaks are observed in the undiluted spectrum ((wavelength, absorbance); (451nm, 4.3); (421nm, 4.2), (416nm, 4.5), (401nm, 4.2), (371nm, 4.6), (341nm, 4.4) & a quasi peak at (482,4.0)). The small peak at 421nm is always expected for diatomic iodine, based on computational work, which is expected to be present in both samples and is confirmed in this data. Note how sharply the absorbance spectra of undiluted iodine changes in the 500600 nm region. Because MAG nascent consumable iodine is typically diluted in water in normal nutritional supplementation, the spectra of RAW and MAG diluted in DW is of interest. Additionally, dilution in alcohol is natural to study since undiluted iodine tincture is dissolved in alcohol already and requires no solvent change for further dilution. Figure 1 shows that two very large UV peaks are observed in both RAW & MAG upon dilution in either DW or SpecA. For DW dilution, RAW peaks are (351nm, 1.74), (288nm, 2.37), and quasi-peaks (~205nm, 4.06) and (~460nm, 0.63). MAG peaks are identical (351nm, 1.68), (288nm, 2.27) having slightly lower absorbance of official peaks and also has similar quasi-peaks (~205nm, 4.06) and (~460nm, 0.65). For SpecA dilution, RAW peaks are (359nm, 0.50), (291nm, 0.87) and UV quasi peak appears red shifted in alcohol compared to water being (~225nm, 0.91), and a broad quasi peak (~460nm, 0.07) is not readily visible on the graph; MAG peaks diluted in SpecA are similar (358, 0.39), (291, 0.69) with similar quasi~peak (~225nm, 0.85) and broad quasi~peak (~460, 0.06) not readily visible on graph either. Neither alcohol nor water have any visible peaks although both interfere with signal slightly below ~205nm (205nm, 0.19) for SpecA and (205nm, 0.07) for DW, and DW contributes a uniform addition to absorbance of ~0.035 abs units (note how iodine in DW dilutions is shifted up slightly on the Y axis).
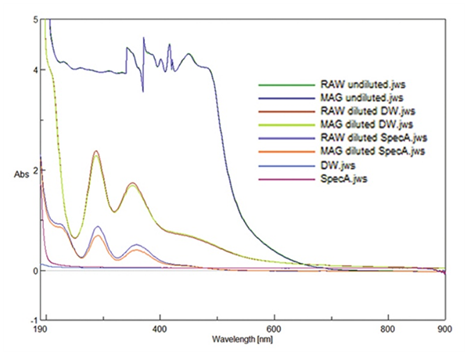
Figure 1 Spectra of Magnascent vs. Raw Iodine. Electromagnetically processed (MAG) and unprocessed (RAW) iodine spectra for comparison, when undiluted, diluted in distilled water (DW), and diluted in spectroscopic grade alcohol (SpecA); spectra of DW and SpecA.
Difference between magnascent & raw iodine
Because differences in wavelength and absorbance between processed and unprocessed iodine are relatively small (Figure 1), higher resolution spectroscopy to the limit of the V~650 spectrophotometer is required to see whether such differences are likely real (due to a real difference in "e" the molar absorptivity due to differing iodine species), or whether they are artifacts (due to small unintended differences in "C," the iodine concentration). Since highly accurate electronic pipettes (Rainin E4-XLS) are used which come with certificates of calibration and conformity, differences in diluted concentrations of MAG or RAW for a particular solvent (DW or SpecA) are expected to be very low. To verify small differences between patented processed iodine and raw iodine, spectroscopy is performed at the optimum resolution of the V~650 instrument with a smaller bandwidth (0.1nm instead of 2nm) which allows for a decreased interval of data collection (data every 0.05nm instead of every 1nm) and the acquisition time was significantly reduced (100nm/min spectrum scan, instead of 2000nm/min). As before, the same cuvette is used to avoid small differences in index of refraction or cuvette manufacturing differences. As before, but with significantly increased resolution, the spectrum of RAW and MAG is obtained for undiluted, diluted in DW and diluted in SpecA samples, and then the RAW spectrum of each is subtracted from the MAG spectrum of each to see the difference (Figure 2). If absorbance is zero at a particular wavelength after subtraction then there is no difference, if absorbance is positive at a particular wavelength then MAG has more absorbance there, and if absorbance is negative then RAW had more absorbance there. As can be seen in Figure 2, RAW has more absorbance for many wavelengths in all samples as the MAG-RAW signal is negative. Note that the undiluted MAG-RAW signal (green) appears to be very noisy in the UV-VIS (<500nm) corresponding to where the undiluted sample has very high absorbance (>4) greater than the linear range of the instrument (compare to undiluted in Figure 1); however, a clear negative difference is seen in the VIS region (~500-645nm); selected values of MAG~RAW undiluted (500nm, ~0.088), (550nm, ~0.025), (600nm, ~0.014), (650nm, ~0.0028). No difference is seen in the IR region (zero absorbance to three significant figures); selected value (700, 0.00022), (750nm, 0.00025), (800, 0.00025). For the iodine dilutions in DW, the MAG-RAW is much less noisy in the UV and shows 3 broad peaks with a maximum difference peak at b/t ~288-292nm (288.3nm, ~0.42), (291.7nm, ~0.43) and b/t ~348~359nm (348.4nm, ~0.30), (358.6nm, ~0.29) as well as a smaller broad peak at ~220-226nm (220.5, ~0.15), (225, ~0.14). Other regions of interest, although difficult to resolve from the noise visually from looking at Figure 2, are tiny local maximum "i.e. more negative" negative difference peaks at (200.45nm, ~0.25) and (205nm, ~0.24). For the iodine dilutions in SpecA, two main negative peaks are seen with a sharp peak at (291.4, ~0.30), and a more broad peak at ~356-364 ((359.9, ~0.18)), and a quasi~peak at ~227nm (226.7nm, ~0.19). Figure 2 shows a readily visible red shift (shift to the right toward the IR) of the SpecA diluted MAG-RAW compared to the DW diluted SpecA (notice the difference in the minimum difference in absorbance (248.35 nm, ~0.045) for DW to (259.1nm, ~0.065) for SpecA diluted difference and from (323.3nm, ~0.16) for DW to (324.8nm, ~0.089) for SpecA diluted difference. Alternatively, this could be viewed as a blue shift (more towards UV) when going from alcohol to water solvent. One region of interest in particular on Figure 2 is (258nm, ~0.067) where the MAG~RAW in DW and in SpecA difference curves intersect. Could perhaps this point represent an "iso-solvent" point useful for future work as a place to monitor changes in nascent iodine (nascent has less absorbance than raw here via the negative difference here) without having to worry about differences in alcohol versus water solvent. Regardless, Figure 2 shows many differences between MAG and RAW iodine and opens up many exciting avenues of further nascent iodine research.
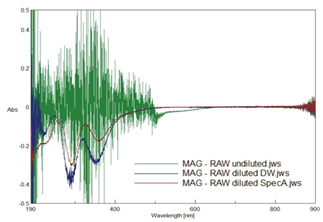
Figure 2 Differences between Nascent & Raw Iodine.
Subtraction of hi-resolution spectra of RAW from MAG: undiluted, diluted in distilled water (DW), and diluted in spectroscopic grade alcohol (SpecA). Multiple differences seen b/t patented nascent iodine and unprocessed raw iodine confirmed in the ultraviolet (<400 nm) and visible (<700 nm) regions. No difference is seen in the infra-red (>700 nm).
Differences in raw and patented electromagnetically processed Magnascent iodine are observable in the UV and VIS spectral region using hi-resolution spectroscopy. MAG is observed to have a lesser absorbance at peaks near (351nm & 288nm) when diluted in DW and more noticeably lesser absorbance at peaks near (359nm & 291nm) when diluted in SpecA. Spectra for MAG & RAW obtained using high resolution spectroscopy are obtained and subtracted (MAG-RAW) and no difference is seen in the IR, but negative difference (lower MAG absorbance) is seen in the VIS ~500-645nm, and negative difference is seen in UV at the two major peaks for samples diluted in water or alcohol (DW: 248.3nm, 323.3nm), (SpecA: 259.1nm, 324.8nm) and the minor peak/quasi peak (DW 205nm), (SpecA ~225nm). This work demonstrates the utility of absorbance spectroscopy in analyzing differences in raw and nascent iodine, and highlights the complexity of liquid iodine analysis and the need for a better understanding of the basic science underlying many potential liquid/solvent iodine species for better understanding of iodine nutritional supplementation.
The tests described and the resulting independent data presented herein performed by iMedDo Inc were supported by research under Magnascent Division of Shield Bearer (John & June Brookshire, Directors) whom author thanks for shared support and enthusiasm for nascent iodine. Permission was granted by Shield Bearer Inc. to publish this information. The findings of this paper are not to be construed as an official Shield Bearer Inc. position unless so designated by other authorized documents. Magnascent® is a registered trademark of Shield Bearer Inc.© Shield Bearer Inc. 2014-2016.
Author has no financial interest to disclose in Shield Bearer Inc or Magnascent®, but is President of iMedDo Inc which sells iodine health supplement NeuIodine™.

©2016 Goins. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.