Journal of
eISSN: 2373-633X


Research Article Volume 4 Issue 4
1Department of Radiology, Akdeniz University, Turkey
2Department of Pathology, Akdeniz University, Turkey
3Department of Otorhinolarynglology, Akdeniz University, Turkey
Correspondence: Kamil Karaali, Department of Radiology, Akdeniz University Hospital, 07059, Antalya, Turkey, Tel 90-242-2496440
Received: February 05, 2016 | Published: March 17, 2016
Citation: Ertem S, Toru HS, Derin AT, et al. Contribution of diffusion-weighted MR images in the differentiation of benign and malign head and neck mass lesions. J Cancer Prev Curr Res. 2016;4(4):120-122. DOI: 10.15406/jcpcr.2016.04.00126
We evaluated the value contribution of diffusion-weighted MR images in the differentiation of benign and malign neck masses. We retrospectively evaluated the findings of 102 patients with neck masses. Mean ADC (apparent diffusion coefficient) values of malignant lesions were 1,27±0,57mm2/s, 95% confidence interval was (1,18 mm2/s - 1,49 mm2/s). Mean ADC values of benign lesions were 1,61±0,67mm2/s, 95% confidence interval was (1,42 mm2/s - 1,84 mm2/s). When we compare these ADC values, mean ADC values of malignant lesions were smaller than those of benign lesions. Result was statistically significant (p<0,01). We concluded that, diffusion-weighted MR imaging may be used to help in characterization of head and neck lesions.
Keywordsmr imaging, diffusion weighted mri, neck masses
MRI, magnetis resonance imaging; DWI, diffusion-weighted imaging; T, tesla; CT, computed tomography; ADC, apparent diffusion coefficient; ROC, receiver operating characteristic; ROI, region of interest
Head and neck cancer is a significant cause of mortality worldwide. Patients treated for advanced head and neck cancer may suffer from severe sequela. Furthermore, head and neck cancer causes high health care cost. Both sequela and health care cost can be diminished with optimized treatment which requires accurate estimation of tumor size and extent. For these purpose accurate discrimination of vital tumor tissue and tumor-free soft tissue is mandatory.
CT and MRI are the two imaging modalities of choice for establishing the diagnosis and for staging head and neck cancer. But with both techniques, sometimes the discrimination of benign versus malignant tissues is difficult. The reason of this is mainly due to the fact that both of these techniques may only evaluate the macroscopic morphology of biologic tissues without reflecting the microstructural differences.
Diffusion-weighted imaging (DWI) is a non-invasive technique which analyzes the structure of a biologic tissue at a microscopic level and may therefore be a potential tool for evaluating the microstructural differences between vital tumor tissue and tumor- free tissue.
In this study our aim was to evaluate whether DWI is a feasible and reliable technique for differentiating benign from malign neoplastic tissue in the patients that reffered to our clinic and suffering from head and neck mass.
We retrospectively investigated all the patients that referred to our clinic because of head and neck mass and searched for tumor recurrence or residue after chemotherapy and/or radiotherapy or surgery. For the last three years, there were 110 patients aged 0-84 years (Mean 47,5). Forty-nine of them were female, 61 were male. Eight patients were excluded because either no pathologic finding determined after routine MRI investigation or easily diagnosed by routine MRI sequences. Therefore, 102 patients were included into the study. Ethics committee approval and informed consent from all the patients were obtained.
MRI was performed using a 1,5 T MRI unit (Simens Avanto, Erlangen, Germany) and dedicated head nad neck coil was used. The Standard MRI protocol for head and neck were used. DWI always performed before application of intravenous contrast. None of the patients had major artifacts at DW imaging that warranted exclusion from the study. For quantitative analysis of tissue-spesific diffusion properties, the ADC map was created by the MRI unit automatically. ADC values were calculated offline on a pixel-by-pixel basis from user defined regions of interest (ROI). The ROI were drawn manually by two experienced radiologist after identifying the tumour on routine head and neck images. As a reference between the patients, ADC values were also measured from spinal cord or medulla oblangata. For each patient routine MR images and ADC measurements were recorded on CDs.
In 90 patients the final diagnosis were base program version 11.3.3.0) to evaluate the diagnostic capability of the ADC value for use in the differentiation between malignant and benign lesions. We made two other comparisons: First, we compared ADC values of the lesions in patients who had no prior therapy, and the patients who had chemotherapy/radiotherapy or surgery. Mann Whitney-U test SPSS program was used for the statistical analysis. Second; we compared ADC values of the lesions that are smaller and larger than 1 cm. For this comparison, we used Mann Whitney-U test SPSS program. Alpha level is accepted as 0,05.Ód on pathologic findings obtained by either biopsy or operation specimens. In 11 patients, the diagnoses were accepted as benign after 6 months of follow-up and no change was observed. In one patient, who had inoperable invasive paranasal sinus tumor (Figure 1), the diagnosis was accepted as a malign lesion. After these assesment 61 patients were diagnosed as malignant, 41 patients were diagnosed as benign. Mean ADC values of these lesions were compared statistically. The distribution of the malignant lesions is shown in Table 1. We used receiver operating characteristic (ROC) curve analysis (medcalc program version 11.3.3.0) to evaluate the diagnostic capability of the ADC value for use in the differentiation between malignant and benign lesions. We made two other comparisons: First, we compared ADC values of the lesions in patients who had no prior therapy, and the patients who had chemotherapy/radiotherapy or surgery. Mann Whitney-U test SPSS program was used for the statistical analysis. Second; we compared ADC values of the lesions that are smaller and larger than 1 cm. For this comparison, we used Mann Whitney-U test SPSS program. Alpha level is accepted as 0,05.
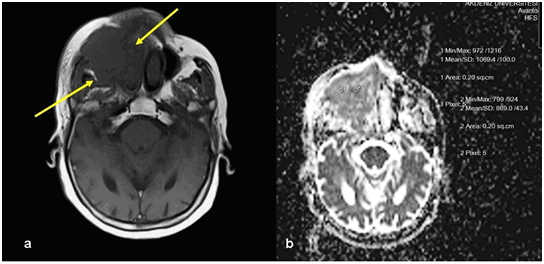
Figure 1 Invasive paranasal tumor. A) Precontrast T1-weighted image show mass lesion within right maxillary sinus (arrows) B) ADC measurement of the same lesion on DWI.
Squamous Cell Carcinoma |
24 |
Nasopharyngeal Carcinoma |
12 |
2 |
|
Malignant Epithelial Tumor |
10 |
Leuchemia/Lymphoma |
3 |
Salivary Gland Type |
1 |
2 |
|
Metastatic Tumors |
2 |
1 |
|
Thyroid Medullary Carcinoma |
2 |
Combined Small Cell Carcinoma, Neuroendocrine Type |
1 |
Total |
61 |
Table 1 Distribution of malignant lesions
Mean ADC values of malignant lesions were 1,27±0,57 mm2/s, 95% confidence interval was (1,18 mm2/s - 1,49 mm2/s). Mean ADC values of benign lesions were 1,61±0,67 mm2/s, 95% confidence interval was (1,42 mm2/s - 1,84 mm2/s). When we compare these ADC values, mean ADC values of malignant lesions were smaller than those of benign lesions. Result was statistically significant (p< 0,01) When the cut off value of ROC curve accepted as 1,72 the specificity was 83,6%, sensitivity was 43,9%. The area under curve was 0,660±0,0558, 95% confidence interval was 0,559 - 0,751, p value: 0,0041 positive likelihood ratio: 2,68 and negative likerlihood ratio was 0,67.
Among the malignant lesions; the smallest ADC value was measured in a case with malignant lympoma (ADC: 0,43mm2/s), the highest ADC value was measured in a patient with embryonic rhabdomyosarcoma (ADC: 2,82mm2/s).
The mean ADC value of the lesions in patients with no prior therapy ( mean: 1,14 mm2/s ; confidence interval: 0,43mm2/s - 1,89mm2/s) were smaller than those of post therapy (chemotherapy and/ or radiotherapy or surgery) group (mean: 1,37mm2/s confidence interval: 0,74 mm2/s - 3,43 mm2/s). But the result was not statistically significant (p: 0.85).
The mean ADC value of lesions larger than 1cm (mean: 1,23 mm2/s confidence interval: 0,43 mm2/s – 2,82 mm2/s) was smaller than those of lesions smaller than 1cm (mean: 1,56 mm2/s confidence interval: 0,49 mm2/s -3,43mm2/s). But the result was not statistically significant (p: 0.129).
Difusion weighted MR images are mainly used for demonstration of cytotoxic edema in early stages of brain infarction.1 However, it may also give valuable information about microstructure of tumors.2 In malign cells, nuclei are larger with high chromatine content. Tissues of malignancy also show hyper cellularity. Those micro structural characteristics result in restricted diffusion of the protons. Tumor cellularity and low ADC relationship can be demonstrated in various kinds of tumors, including head and neck tumors.3 Increased nucleocytoplasmic ratios usually result in restricted diffusion. Diffusion weighted MRI is relatively fast method and does not require contrast material injection, therefore, it can be added to routine sequences.
Friedrich et al.4 have used diffusion weighted MRI sequences with 800 s/mm2 b values and they have obtained sufficient images for the dsicrimination of tumoral and non-tumoral tissues in the head and neck squamous cell cancers4 In our study we have also used 50 and 800s/mm2 b values for diffusion weighted sequences.
Wang et al., evaluated ADC values of head and neck lesions and found that malign lesions have lower than benign lesions, also, they stated that malignant lymphomas have lower ADC values than carcinomas. As well, they found that benign cystic lesions have higher ADC values than benign solid lesions5 they found 91 % specificity and 84 % sensitivity if the cut-off value is set to 1.22x103mm2/s.
Vandecaveye et al.6 have found that malignant lyph nodes in the neck have significantly lower ADC values than benign nodes. When the cut-off value was set to 0.94x10-3mm 2/s, sensitivity was 84 % and specificity was 94 %.6 In our study, we also evaluated the lymph nodes by comparing the ADC values of those smaller and larger than 1cm but we could not find significant difference. However, the number of lymph nodes in our study was not too many and heterogeneity of the primary lesions may be the cause of non-significant results for the lymph nodes.
Maeda et al.7 have stated that DWI may be helpful in the differentiation of carcinomas and lyphomas in malignant group, and necrosis and abscess in benign group of head and neck lesions. However, they emphasized that ADC may be influenced by many factors like yumor celluratiy and matrix and ADC measurements may sometimes be confusing7 in our study, we also observed a similar overlap of the results obtained from benign and malignant group.
Razek et al.8 evaluated the masses in pediatric head and neck lesions with DWI and have found that in malignant lesions, mean ADC was 0,93x10-3mm2/s, 1,57x10-3 mm2/s in benign solid lesions and 2,01x10-3 mm2/s in benign cystic lesions. The difference was significant between benign and malignant group.8
Tumor necrosis and cystic degeneration may decrease the restricted diffusion of malignant masses. It is recommended that, ADC measurement should be performed from enhancing solid portions of tumors.8 However, very small necrosis areas may also be present and result in higher ADC values of malignant masses. Sumi et al.9 have found that metastatic lymoh nodes have higher ADC values than benign lymph nodes. This was explained by the presence of necrosis within the lymph nodes9 In our study, highest ADC value observed in malign masses was 2.82mm2/s in a embryonal rhabdomyosarcoma metastasis and we thought that this was due to intrinsic necrosis (Figure 2).
In conclusion, diffusion-weighted MR imaging may be used to help in characterization of head and neck lesions. Since the DW MRI sequence is fast and effective, it should be a part of routine head and neck imaging protocol in clinical practice. However, it should be kept in mind that measureöents should be made from the solid portions of the lesions. As well, overlapping results may still be observed and DWI should only be used as additional information to conventional sectional images.
None.
None.
The authors declare no conflict of interest.

©2016 Ertem, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.