Journal of
eISSN: 2373-633X


Case Report Volume 3 Issue 1
Prince Sultan Oncology Center, King Salman Armed Forces Hospital, Saudi Arabia
Correspondence: Ehab Hanafy, Prince Sultan Oncology Center, King Salman Armed Forces Hospital, 71411 Tabuk, Kingdom of Saudi Arabia, Tel 9.66503E+11
Received: August 12, 2015 | Published: August 26, 2015
Citation: Pakra MAI, Hanafy E. Sacrococcygeal atypical teratoid/ rhabdoid tumor in an infan. J Cancer Prev Curr Res. 2015;3(1):222-225. DOI: 10.15406/jcpcr.2015.03.00070
We present a case of Atypical Teratoid/ Rhabdoid Tumor (AT/RT) arising from the sacrum in an 8 months old infant, who was given chemotherapy and had total surgical excision of the tumor. It is well-known that tumors arising from the sacrococcygeal area are very rare and most of them are metastasis derived from lung, breast, kidney, prostate, head and neck, gastrointestinal or skin cancers, and to our knowledge this is the first documented case of AT/RT arising from the sacrococcygeal area in an Infant.
Keywords: atypical teratoid/rhabdoid tumor, sacrococcygeal tumors, rare tumors, brain tumors, CNS tumors
Atypical Teratoid/Rhabdoid Tumor (AT/RT) is a very rare, fast-growing tumor of the brain and spinal cord. It usually occurs in children younger than three years of age. The tumor has very poor prognosis even with variety of chemotherapy, surgery with or without radiotherapy. Sacrococcygeal tumors are very rare, they include benign or malignant neoplasms, and on review of the literature, we found no reported cases of AT/RT arising from the sacrum.
A.A. is a 2 years old boy who presented at the age of 8 months when the mother noticed a firm, non-tender swelling on the sacral area. The child had no symptoms or signs of lower limb affection, however he had urine incontinence, other systems review was unremarkable. The child’s developmental history is normal for age, no perinatal history of concern, his vaccination is up to date and he is not known to have any drug allergy. He was first seen by the Neuro-surgery Team, when CT Figure 1 and MRI pelvis and sacrum(Figure 2 were requested and showed a large (25.5 X 34.8 X 21.8 mm) soft tissue mass arising from the lower 3 sacral segments and the coccyx. It had a large retro vertebral component, which could not be separated from the left gluteus maximum muscle. It filled the spinal canal with posterior vertebral scalloping, and had a small anterior intra pelvic component without apparent line of cleavage from the posterior rectal surface. It contained small hyper-densities representing bony remnants and calcification. As a result; the child underwent incomplete surgical excision of the swelling.
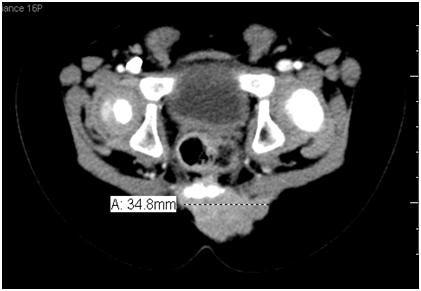
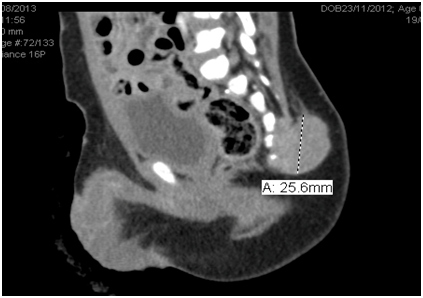
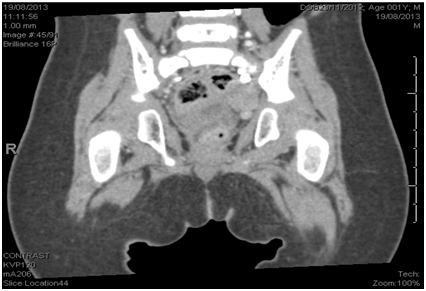
Figure 1 CT Pelvis and Sacrum: A large (25.5 X 34.8 X 21.8 mm) mass is seen arising from lower 3 sacral segments and the coccyx.
Histopathology studies showed a poorly differentiated malignant neoplasm with diverse immunopositivity; differential diagnosis included mixed germ cell tumor (Germinoma +Yolk sac tumor with aberrant immune expression), malignant Rhabdoid tumor and Melanocytic malignant neuroectodermal tumor of infancy.
The child was referred to Pediatric Oncology department when staging work up was done. CT chest was normal and Bone Scan was unremarkable. We started the child on PEB (Cisplatin, Etoposide, Bleomycin) for two cycles depending on the initial histopathology and we evaluated him by CT scan that showed only minimal decrease in the tumor size. Histopathology was reviewed again, it favored the diagnosis of Atypical Teratoid/Rhabdoid Tumor, and the result was seconded by an external reference laboratory (Figure 3A–3D).
The patient was started on the European Rhabdoid Registry protocol. He received 2 cycles of (DOX – ICE – VCA) (Table 1). Following the 2 cycles of chemotherapy the child had MRI pelvis and Sacrum done, which showed further decrease in the tumor size.
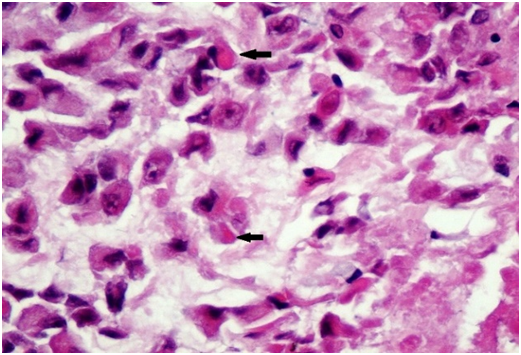
Figure 3A Malignant cells with eccentric nuclei and eosinophilic cytoplasmic inclusions (arrows), HE x20.
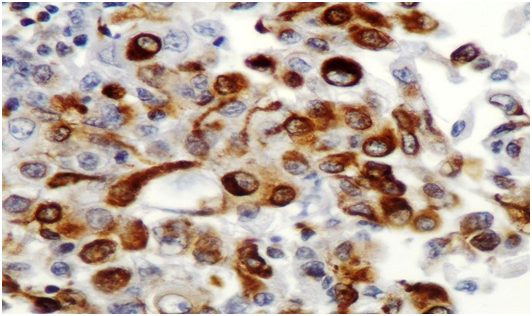
Figure 3D Immunohistochemistry, Vimentin x40.
Figure 3 Microscopic examination showed a solid tumor with focal papillary architecture, the malignant cells had large vesicular nuclei with prominent nucleoli; their cytoplasm was abundant containing PAS positive inclusions and vacuolization. There was moderate mitotic activity and diffuse apoptosis. The tumor cells showed loss of INI1, positivity for Vimentin, EMA and high proliferation index (60%), whereas Desmin, MSA, S100, AFP, MyoD1, MDM2, OCT4 & CD30 were negative.
DOX |
|
Doxorubicin |
37.5 mg/m2 D1,D2 |
ICE |
|
Ifosfamide |
2 gm/m2 D1,2,3 |
Carboplatin |
500 mg/m2 D1 |
Etoposide |
100 mg/m2 D1,2,3 |
VCA |
|
Vincristine |
1.5 mg/m2 D1,8 |
Cyclophosphamide |
1.5 gm/m2 D1 |
Actinomycin-D |
25 microgram/kg D1,2 |
Table 1 Chemotherapy regimen & doses of one cycle
The child was referred back to the neurosurgery when a total surgical excision of the tumor was achieved and the Histopathology review confirmed the diagnosis of AT/RT. We continued one more cycle of chemotherapy (DOX – ICE – VCA) after which MRI Sacrum and Pelvis showed complete remission of the tumor Figure 4. Following that, the child started his regular follow up in our oncology center.
Atypical Teratoid/Rhabdoid Tumor (AT/RT) is a very rare, aggressive tumor of the brain and spinal cord. It usually occurs in children younger than three years of age, although it can occur in older children and adults. About 60% will be in the posterior canal fossa (particularly the cerebellum). It was estimated in one review to be 52% posterior fossa, 39% sPNET (supratentorial primitive neuroectodermal tumors), 5% pineal, 2% spinal, and 2% multi-focal.1
Tekautz TM et al.,2 reported only one case of AT/RT of the spine out of 31 patients diagnosed with CNS AT/RT, during a 19-year period.2 Furthermore, Review of the documented cases of spine AT/RT in the literature revealed an age range from 2 months to 9 years and none of which occurred in the sacroccoygeal area.3
Gross feature of AT/RT resembles medulloblastoma and sPNET. They are soft, pale pink tumors with areas of hemorrhage as well as necrotic regions. Rhabdoid cells are characterized by eosinophilic cytoplasm, large nuclei with excentric nucleoli and a prominent membrane as well as cytoplasmic eosinophilic inclusion bodies is seen. These diagnostic cells may be grouped in nests close to areas composed of neuroectodermal, mesenchymal or epithelial tissue types. Only about 10 to 15% of AT/RT consists almost exclusively of rhabdoid cells. AT/RT exhibits a broad spectrum of immunohistochemical reactions corresponding to the different tissue subtypes. Rhabdoid cells express vimentin, EMA (epithelial membrane antigen) and cytokeratin, less commonly SMA (smooth muscle actin). Lost INI1 protein expression by immunohistochemical studies in the tumor cells is a strong indicator for AT/RT, however, rare AT/RT with preserved INI1 expression are also on record.4
Radiological findings in spine AT/RT are nonspecific but are almost the same as described in the brain. The lesions are heterogeneous, reflecting their histopathologic complexity. Other imaging features include isointensity/hypointensity to cord on T2-weighted sequences and contrast enhancement.5
Therapeutic approaches include surgery, postoperative chemotherapy regimens with or without radiation therapy.6 There is only limited data from occasional survivors with spinal AT/RT. Published series on CNS AT/RTs report an overall 2-year survival rate of less than 15% for children younger than 3 years at diagnosis.7 On the other hand, Tumors arising from the sacrococcygeal area are very rare. Most common are metastasis derived from lung, breast, kidney, prostate, head and neck, gastrointestinal or skin (melanoma) cancers.8
Primary benign and malignant tumors of the sacrum may arise from bone or neural elements, or the bone marrow in cases of hematological malignancies. Ten percent of all benign bone tumors and six percent of all malignant bone tumors involve the sacrum (Table 2). Other benign and malignant tumors of the sacrum are exceptional.9 Patients with sacral tumors present with nonspecific symptoms, like pain, palpable mass and neurologic deficits. The aim of CT and MR imaging is to define the anatomic origin, extent, and radiologic features of a given lesion. Moreover, sacral tumors have characteristic features on CT scans and MR imaging that may aid in making a diagnosis.10
Benign tumors |
Malignant tumors |
||
Giant cell tumors |
60% |
Chordomas |
50% |
Aneurysmal bone cysts |
4% |
Lymphomas |
9% |
Osteoblastomas Multiple myelomas |
9% |
Ewing’s sarcoma |
8% |
Table 2 Tumors of the sacrum
We herein report the first case of Atypical Teratoid/Rhabdoid Tumor arising from the sacral spine in 8 months old boy. We preferred to give the child preoperative chemotherapy including a variety of chemotherapeutic agents which seemed to be effective in reducing the size of the tumor, thus facilitating a total surgical excision.
None.
The authors have no financial conflicts of interest to declare.
None.

©2015 Pakra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.