Journal of
eISSN: 2373-4396


Conceptual Paper Volume 13 Issue 1
1Interventional Cardiologist,Alborz University of Medical Sciences, Iran
2Department ofEchocardiographist, Cardiologist,Alborz University of Medical Sciences, Iran
Correspondence: Mehdi Mousavi MD, Interventional Cardiologist, Alborz University of Medical Sciences, Shaheed Rajaei Hospital, Karaj, Iran, Tel +989123053284
Received: December 07, 2019 | Published: January 3, 2020
Citation: Yazdani S, Mousavi M, Joghataie P. Resolution of a high risk AV prosthetic valve malfunction after fibrinolytic therapy: a case report. J Cardiol Curr Res. 2020;13(1):1-5. DOI: 10.15406/jccr.2020.13.00462
Introduction: Prosthetic valve thrombosis (PVT) may be life-threatening if left untreated. History (recent change in symptoms including dyspnea), physical examination (recent change in prosthetic heart sounds), and different available imaging modalities including transthoracic (TTE) and transesophageal echocardiography (TEE) and fluoroscopy could lead to the diagnosis. Depending on prosthetic valve position, thrombosis size and patient’s symptoms, a range of therapeutic options including an intensification of anticoagulation, fibrinolytic therapy, and surgical intervention exist.
Case presentation: A 51-year-old woman with a history of aortic valve replacement (AVR) 9years ago was admitted with refractory pulmonary edema and function class IV dyspnea. TTE showed severe left ventricular systolic dysfunction with an ejection fraction of 10-15% and a mean transvalvular aortic valve (AV) gradient of about 20mmHg. Fluoroscopy revealed prosthetic valve malfunction with one leaflet being fixed. TEE confirmed the etiology with a 0.9cm2 thrombosis. Due to high surgical risk and refusal of surgery by both patient and surgeon, fibrinolytic therapy with streptokinase was started. Improved patient's symptoms, decreased mean AV gradient to 7mm Hg and improved valvular motion in fluoroscopy were documented after the treatment, with severe abdominal pain as a presumed complication.
Discussion: In spite of current guideline recommendation for surgical treatment in patients with left-sided PVT who are in function class III and IV or who have large clot burden, still fibrinolytic therapy could be considered as a possible treatment option in extremely high-risk patients.
Keywords: aortic valve replacement, prosthetic valve thrombosis, fibrinolytic therapy, anticoagulation
PVT, prosthetic valve thrombosis; TEE, transesophageal echocardiography; CT, computed tomography; AVR, aortic valve replacement; NYHA, New York heart association; INR, international normalized ratio; CCU, cardiac care unit
Many patients with valvular heart disease are treated with prosthetic valves in daily practice. A range of well-known complications may occur after the implantation of these prosthetic valves during follow up. Among the causes of prosthetic valve malfunction, prosthetic valve thrombosis (PVT), especially in mechanical valves, is a very serious condition, associated with high morbidity and mortality. Prosthetic valve thrombus formation is usually the direct result of the thrombogenic surface of the prosthesis, however inadequate anticoagulation (the most important factor), change in trans-prosthetic blood flow, left atrial function, or loss of effective atrial contraction may also have a role.1‒3
Successful treatment requires a rapid diagnosis. A high level of clinical suspicion is needed to diagnose prosthetic valve thrombosis (PVT) in a patient with an altered medical condition.4 Disappearance or attenuation of the prosthetic valve sound in physical examination or noticed by the patient may be an important clue to the diagnosis. Dyspnea also has been reported to be the predominant symptom at presentation.5,6 Transthoracic (TTE) and transesophageal echocardiography (TEE), as well as fluoroscopy, are the diagnostic procedures of choice for the detection of PVT.7‒9 2017 AHA/ACC focused update guideline recommend an evaluation with multimodality imaging with TTE, TEE, fluoroscopy, and/or computed tomography (CT) scanning in patients who are suspected to malfunction of the mechanical prosthetic valve due to thrombosis. This multimodality approach is presumed to be more effective than a single imaging modality in detecting and characterizing valve thrombosis in terms of valve function, leaflet motion, and extent of thrombus.10 After the detection of the PVT, the next challenge would be the selection of the most appropriate therapeutic approach. In small thrombosis with no hemodynamic compromise and stable clinical condition, a chance with an intensification of anticoagulation would be warranted. In PVTs with clinical instability or significant valve dysfunction in echocardiography and fluoroscopy, the therapeutic approach depends on valve position, thrombosis size, degree of valve malfunction and patient’s clinical condition.7,9,11‒14 We, herein, present a challenging case of aortic PVT with concomitant severe left ventricular systolic dysfunction.
A 51-year-old woman with a history of aortic valve replacement (AVR) with Saint Jude prosthetic valve, 9years ago was admitted for the New York heart association (NYHA) function class IV dyspnea as well as orthopnea and paroxysmal nocturnal dyspnea. The patient complained of progressive dyspnea since 9months ago for which she had been admitted several times and received medical treatment. Several transthoracic echocardiograms (TTE) were performed, but no fluoroscopy was performed during previous admissions. On physical examination blood pressure was 100/60mmHg, heart rate was 100/min and respiratory rate was 20/min. The metallic sound of the prosthetic valve was inaudible, and crackles were heard up to 2/3 of both lungs and the patient had severe edema in both lower extremities.
The patient was under treatment with warfarin (4days/week: 5mg/das and 3days/week: 2.5mg/day), furosemide, spironolactone, digoxin, ASA, carvedilol, atorvastatin, and insulin (for diabetes).The initial laboratory evaluation showed prothrombin time (PT) of 25seconds with an international normalized ratio (INR) of 2.95, creatinine of 0.7mg/dl, hemoglobin of 9.1g/dl, and blood sugar of 161mg/dl (8.95mmol/l). The electrocardiogram showed sinus tachycardia with left bundle branch block.
The initial transthoracic echocardiography (TTE) showed an ejection fraction of about 15%, moderate to severe right ventricular dysfunction, (TAPES, 12mm), and significant pulmonary hypertension with systolic pulmonary arterial pressure of about 55mmHg. Hemodynamic study of AV prosthesis showed mean AV gradient of 20mmHg, an acceleration time of 115milliseconds and DVI (LVOT VTI/AV VTI) of about 0.17. It also showed small moving particles on the ventricular side of the aortic valve, mostly suggestive of a clot. The patient was admitted to the cardiac care unit (CCU) and initially managed for pulmonary edema as well as heart failure using an infusion of furosemide. The patient’s symptoms improved by 2days and the patient was transmitted to the ward with the termination of her infusions, but one day after discontinuation of her infusions the patient’s dyspnea worsened and we had to transfer her to CCU and start infusions again (Figure 1).
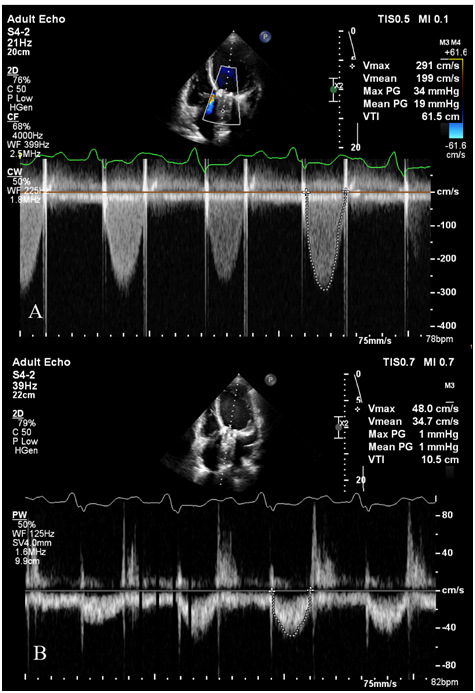
Figure 1 The initial transthoracic echocardiography of the patient showing A. prosthetic aortic valve (AV) mean gradient and VTI; B. Left ventricular outlet (LVOT) mean gradient and VTI. The DVI (LVOT VTI/AV VTI) was: 0.17.
Suspecting to aortic prosthetic malfunction, despite relatively low AV mean gradient, the patient underwent fluoroscopy, which showed the complete fixed motion of one of the AV prosthetic valves leaflets and restricted motion of the other one (Figures 2A&2B). Transesophageal echocardiography (TEE) also confirmed malfunction of the prosthetic aortic valve as well as a 0.9cm2 moving particle on the prosthetic leaflets suggesting a clot.Consultation for cardiac surgery was done. According to cardiac surgery consult, regarding the patient's clinical condition, repeat thoracotomy was a very high risk; mainly due to severe left ventricular dysfunction, and surgeons suggested nonsurgical management and later cardiac transplant if feasible. Furthermore, the patient also refused any surgical intervention.
Considering the patient's high surgical risk and refusal of the surgical intervention, frequent recurrences of the symptoms despite full medical treatment and the clot size we decided to treat the patient using fibrinolytic therapy. Administration of warfarin was hold and intravenous heparin was initiated. When the INR decreased below 2, about 10days after admission of the patient informed written consent was taken and streptokinase was started with the initial dose of 250,000units and then an infusion of 100,000unit/h continued.
Unfortunately, after 8hours of infusion, the patient developed severe abdominal pain around the umbilical region with no apparent tenderness in physical examination. According to consultation with our internists, mesenteric ischemia was suspected due to a mismatch between the patient’s symptoms and physical findings. Peripheral embolization after initiation of thrombolytic therapy especially in the setting of large left-sided prosthetic valve thrombosis was a concern for us and hence the infusion was prematurely stopped, but heparin infusion resumed. Later, ultrasonography of the abdomen was normal except mild ascites.
Fortunately, the patient’s symptoms of dyspnea and orthopnea dramatically improved and the day after the initiation of the streptokinase, the patient became completely symptom-free. Repeated fluoroscopy (Figures 2C&2D) 2days after treatment with streptokinase, showed the normal motion of two leaflets. Repeated TTE also confirmed that the mean AV gradient decreased to 7mmHg. The abdominal pain relieved without intervention and the patient was discharged 4days later uneventfully, with a higher INR goal about 3.5.
Intravenous heparin therapy, fibrinolytic prescription and surgical treatment are considered in the management of prosthetic valve thrombosis. The 2014 ACC/AHA guideline7 and 2012 American College of Chest physicians' guidelines,15 recommended that in cases without contraindication for surgical therapy, NYHA class III and IV is an indication of urgent surgery for left heart prosthetic valve thrombosis (class I, level of evidence B).7 Surgery was also recommended if TEE shows mobile or large thrombus on left heart prosthesis (>0.8cm2) (class IIa recommendation, Level of Evidence: C).7Fibrinolytic therapy was recommended in thrombosed right-sided as well as NYHA class I to II symptoms in a recent onset (<14days) thrombosed left-sided prosthetic heart valve, and a small thrombus burden (<0.8cm2) (class I recommendation with Level of Evidence: B).7 The most recent updated guideline from ACC/AHA, however, recommends urgent treatment using either fibrinolytic therapy or emergency surgery for left sided mechanical heart valve thrombus (Class I, level of evidence: B).10
Our patient with NYHA class IV dyspnea, the clot area of 0.9cm2on TEE and resistant heart failure and pulmonary edema due to malfunction of the prosthetic valve could be a potential candidate for surgical therapy. Nevertheless, very low ejection fraction, as well as repeated surgery, could potentially render the patient very high risk for another operation. Moreover, the refusal of the patient made surgery an impossible option for her treatment.
Considering refusal of the surgery by the patient and surgeon, the 2017 updated guideline,10 the 1997 consensus conference on prosthetic valve11 and some other available data in the literature,12 that indicate thrombolytic could be considered as a treatment option for critically ill patients in NYHA class III or IV, we decided to start thrombolytic therapy as a life-saving treatment and achieved an acceptable response.Fibrinolytic therapy has an 80% effectiveness9 which may be even more in the setting of non-obstructive thrombosis with NYHA class I or II.14 Some suggested regimens for fibrinolytic therapy in prosthetic valve thrombosis are recombinant tissue plasminogen activator (rtPA), urokinase, and streptokinase.13,16‒18 However, complication rate has been high with a reported thromboembolism rate of about 13% and a major bleeding rate of 6% during the years before 2013. Instead, recent experience with slow- infusion of low-dose fibrinolytic has reported a more success rate of 90% with fewer embolic events and major bleeding of <2%.10,19,20 This success rate also is high even in those with larger thrombi and worse NYHA functional class.10 Due to these recent findings19,20 the updated guideline recommends either surgery or fibrinolytic therapy as an urgent intervention in symptomatic thrombosis of a prosthetic valve. some suggested criteria that are in favor of choice of fibrinolytic therapy include: no surgical expertise available; high surgical risk; no contraindication to fibrinolysis; first-time episode of valve thrombosis; NYHA class I–III; small clot (#0.8cm2); no left atrial thrombus; no other valve disease; thrombus visualized versus possible panus; no coronary artery disease (CAD versus concomitant CAD in need of revascularization; and finally the patient choice.10
Attenuation or muffling of the prosthetic valve sound in our patient, as well as progressive refractory dyspnea, were important clues to the malfunctioning prosthetic valve. The most commonly used diagnostic tool for the initial assessment of prosthetic valve malfunction is TTE.8 TTE is helpful for assessment of hemodynamic severity and follow up after resolution of valve dysfunction and according to the most recent American College of Cardiology/American Heart Association (ACC/AHA) guideline in year 2014, is a class I (Level of Evidence: B) indication for assessment of patients with suspected prosthetic valve thrombosis.7 Increase in trans-prosthetic pressure gradient, reduction in the effective valve orifice area, presence of thrombotic mass stuck to the prosthesis and change in the flow pattern may be suggestive for prosthetic valve thrombosis.9
Our patient had a mean pressure gradient of 20mmHg, which is not much high for a patient with a prosthetic valve. Normal functioning prosthetic aortic valve may have a higher gradient, particularly in some settings such as small size valve,21 high stroke volume or pacemaker.22 In the presence of significant LV dysfunction, malfunctional aortic valve prosthesis may just mildly increase in the trans-valvular gradient.21,22 Therefore, differentiation between normal and abnormal valve function in these conditions by only using a transvalvular gradient as the only parameter might be misleading. Consequently, for evaluation of prosthetic valve malfunction indices that are less flow-dependent including acceleration time (AT) and DVI could be more helpful.22 Prolonged acceleration time (AT>100milliseconds and23 low DVI value (less than 0.25)24 should warrant clinical suspicion of valve mulfunction. Considering the increased ratio of DVI and prolonged acceleration time, in our reported patient, we were suspected of a new valvular malfunction.
More accurate details can be provided by TEE25,26 and it is recommended to assess thrombus size and valve motion in order to help making treatment decisions in left heart prosthetic thrombosis (class I, Level of Evidence: B).7 Our TEE confirmed our diagnosis and showed a relatively large clot of 0.9cm2.Fluoroscopy is also helpful to assess valve leaflet motion in patients with suspected valve thrombosis (class IIa indication, Level of Evidence: C).7 While it does not provide hemodynamic data, fluoroscopy is noninvasive and fairly available for prompt evaluation of leaflet mobility and motion of the ring.27 Dealing with an aortic prosthetic valve, if mean pressure gradient is higher than 20mmHg, TTE will play a key role in the diagnosis of malfunctioning valve, but in the absence of this high mean gradient, which could happen in the setting of low cardiac output, fluoroscopy would play an important role.21 Moreover, fluoroscopy might be superior to echocardiography in the study of disc motion in the aortic position.27 Thus, fluoroscopy should be considered as a complementary evaluation to the data obtained by echocardiography.10,21,28 In our patient as discussed above, fluoroscopy was helpful and essential in confirming valvular dysfunction. Fluoroscopy is generally performed in the supine position, and firstly, the postro-anterior and lateral projections are carried out to verify the orientation of the valve.27 Optimal projections are those which allow proper visualization of leaflet motion to calculate the opening and closing angle of the prosthetic valve accurately. These two angles are referred as the measured angle between the two discs in the open and closed positions respectively.21
Despite a minor complication of thrombolytic therapy (a possible emboli), that relieved without any major consequence and intervention, the final result was excellent and life-saving, and we had a successful experience with thrombolytic therapy in a prosthetic valve thrombosis with a clot diameter of 0.9cm2 (>0.8cm2) in a patient with NYHA class IV symptoms. Therefore, we recommend that the management of such high risk patients should be individualized based on a multidisciplinary decision making team involving cardiac surgeon, cardiologist and more importantly, the patient. Fibrinolytic therapy could be considered as a substitute for surgery and the choice could be based on surgical expertise and clinical experience.
This case report highlights that fibrinolysis stands as an effective and life-saving alternative in the treatment of prosthetic valve thrombosis in extremely high risk patients for surgery or those who refuse surgical treatment.
The authors would like to acknowledge Dr. L. Ebrahimi for her help in the drafting of this case report.
The author declares that there is no conflict of interest.
None.

©2020 Yazdani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.