Journal of
eISSN: 2373-4396


Research Article Volume 15 Issue 2
1Division of Cardiology, Department of Internal Medicine, Jikei Daisan Hospital, Japan
2Division of Cardiology, Department of Internal Medicine, The Jikei University School of Medicine, Japan
Correspondence: Takahiro Shibata, Division of Cardiology, Department of Internal Medicine, Jikei Daisan Hospital, 4-11-10 Izumihoncho Komae City, Tokyo 2018601, Japan, Tel +81-3-3480-1151, Fax +81-3-3480-6688
Received: March 31, 2022 | Published: April 4, 2022
Citation: Shibata T, Fukuro E, Takahashi H, et al. Relationship between the efficacy of tolvaptan and hemoglobin levels in acute decompensated heart failure. J Cardiol Curr Res. 2022;15(2):45-51. DOI: 10.15406/jccr.2022.15.00550
Objective: The purpose of this study is to investigate the factors that influence the effectiveness of tolvaptan treatment for acute decompensated heart failure.
Methods and patients: This retrospective study included 102 patients with acute decompensated heart failure who were considered to require tolvaptan. We investigated whether tolvaptan administration was completed within 7 days or more and divided patients into two groups accordingly (responders, n = 35, non-responders, n = 67). Univariate, multivariate analysis and structural equation modeling were used to investigate the various clinical features involved in the success or failure of tolvaptan administration within 7 days. Among the investigation of various factors, changes in blood urea nitrogen, creatinine, and hemoglobin before and after tolvaptan administration were associated with the completion of tolvaptan administration. In order to proceed with further examination, we examined using a path diagram based on structural equation modeling.
Results: It was found that low hemoglobin before treatment and high hemoglobin after treatment are related to the success of the completion of tolvaptan. Other factors were not related to the success of tolvaptan administration. The effectiveness of tolvaptan in pretreatment patients with low hemoglobin is especially important and hemoglobin level will be a valuable marker.
Conclusion: This study showed that tolvaptan may be more effective at low hemoglobin in acute decompensated heart failure, which is generally difficult to treat. In that case, active use of tolvaptan is recommended.
Keywords: Decompensated heart failure, hemoglobin, tolvaptan
The use of diuretics is well established as a treatment for heart failure. In acute heart failure, loop diuretics are generally the first treatment option and are used to reduce the blood volume to treat pulmonary congestion and edema.1 However, the long-term use of loop diuretics can exacerbate chronic heart failure due to overstimulation of the renin-angiotensin-aldosterone system (RAAS).2,3 Not only increased RAAS stimulation but also oxidative stress increase hypertrophy of the renal distal tubular cells, which are associated with the pathogenesis of chronic kidney disease.4,5
Tolvaptan is a drug with the unique properties of an aquaretic diuretic. The inhibition of the vasopressin type 2 receptor by tolvaptan can induce specific water secretion from distal tubules and collecting ducts with relatively lower RAAS stimulation.3 For patients hospitalized with heart failure, tolvaptan treatment has been shown to have no adverse effect on long-term mortality or heart failure-related morbidity,6,7 and recent reports suggest that it can effectively improve the short-term symptoms, signs, and prognosis of acute heart failure.8-13 However, the acute effect of tolvaptan is not applicable for all cases, as the protocols and evaluation methods used are different, because some cases with acute decompensated heart failure have not sufficiently improved with the use of tolvaptan. At present, the Japanese Guidelines suggest loop diuretics as the first recommendation for treating acute decompensated heart failure.14 Tolvaptan is recommended for patients with severe heart failure and renal dysfunction who have stopped responding to loop diuretics. Following to the guideline, in our department, when a patient with acute decompensated heart failure is hospitalized, loop diuretics are the first form of treatment. If the doctor determines that the patient has a poor response to loop diuretics, tolvaptan treatment is initiated to ensure that patients are out of a critical condition as soon as possible.
In some cases, tolvaptan reacts early and the condition improves quickly, so treatment with tolvaptan may be completed within a few days. On the other hand, the effects of tolvaptan may not be fully exhibited. Even in that case, tolvaptan is often used continuously. Because there are not always other alternatives. Therefore, patients who cannot complete tolvaptan treatment tend to have a poor prognosis. For severe heart disease and renal disease, the difference in tolvaptan efficacy between patients appears to be large. Currently, the difference in clinical characteristics between patients who can complete tolvaptan treatment early (within a few days) and those who cannot complete it is not always clear.
It is arguable how many days are appropriate to confirm the acute effects of tolvaptan. However, it is generally considered that about 7 days is a reasonable period.8 We thought that the index of whether tolvaptan could be discontinued within 7 days could be used to characterize the group of cases in which tolvaptan appears to be effective. The purpose of this study was to investigate the factors that influence the success of tolvaptan treatment for acute decompensated heart failure using a variety of statistical methods.
Study population
Of the 345 patients admitted to Jikei Daisan Hospital for acute decompensated heart failure between September 2014 and July 2016, 102 were treated with tolvaptan within 24 hours of admission, because loop diuretics alone were not sufficient to treat severe congestion. These patients had acute decompensated heart failure and/or renal failure and were considered to have a fatal condition or to require mechanical support. Therefore, it was mandatory for doctors to add tolvaptan to the treatment of these patients. This study has been approved by the ethical board of our facility (No. 30-169).
Termination or continuation of tolvaptan treatment
Tolvaptan treatment was discontinued as soon as the symptoms and signs of heart failure improved, after which treatment with loop diuretics was continued. However, if heart failure did not improve sufficiently, tolvaptan treatment was continued. As a result, tolvaptan was used for an average of 168 days (1–1021 days). A previous report8 examined whether tolvaptan treatment could be discontinued within 7 days after it was first administered. In our study, patients were classified into two groups according to whether tolvaptan treatment was discontinued within 7 days (group A: n=35) or more than 7 days (group B: n = 67). Therefore, we decided that the former group contained patients who had responded to tolvaptan and the latter group contained non-responders.
The observation period was 1021 days. Twenty-nine patients (28%) died during this period (group A, n=12; group B, n=17). This difference was not statistically significant. The incidence of dilated cardiomyopathy in group A was significantly lower than that in group B (p < 0.05). There were no significant differences in the numbers of patients with ischemic heart disease, valvular heart disease, type-2 diabetes mellitus, or hypertension. Some cases in which tolvaptan had been used for chronic heart failure were included in group B. Patients undergoing hemodialysis, with acute myocardial infarction and patients under 20 years of age were excluded from the present study.
Vital signs, blood sampling and measurements
Vital signs, body weight, urine volume, systolic blood pressure, and cardiothoracic rate (CTR) on chest radiography were obtained at admission (baseline) and 7 days after admission (on day 7). Routine blood sampling, including blood counts and biochemistry, was performed baseline and day 7. B-type natriuretic peptide (BNP) levels were determined by an enzyme immunoassay using an antibody agonist human BNP (Shionogi Co. Ltd., Tokyo, Japan). Differences in the admission and day 7 values were calculated as the change in the range of each factor, expressed as delta (Δ), where
Delta (Δ) = (day 7 value) – (baseline value).
Left ventricular ejection fraction on echocardiography
At the time of admission, all patients underwent transthoracic echocardiography using an echo machine (Vivid E95, GE Health Care Japan, Tokyo, Japan). Conventional variables (left ventricular diastolic dimension, left ventricular systolic dimension, left ventricular inflow velocity and left ventricular outflow velocity) including the left ventricular ejection fraction (LVEF), were measured. The LVEF was obtained using the modified Simpson method and the trace end systolic and end diastolic dimensions were calculated.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation, and categorical variables were expressed as percentages. Continuous data were analyzed using Student’s t-test, and categorical data were analyzed using the chi-squared test. Univariate and multivariate logistic regression analyses were performed to identify the clinical factors (age, blood urea nitrogen, creatinine, hemoglobin and LVEF) influencing the completion of tolvaptan (drug) administration (CDA) before and after treatment. These statistical analyses were performed using the SPSS software program (version 21.0; SPSS Inc., Chicago, IL, USA). P-value of < 0.05 were considered to indicate statistical significance.
In addition to univariate and multivariate analyses, we performed structure equation modeling.
This is because structure equation modeling can remove as many confounding factors as possible. Furthermore, it is possible to examine whether the absolute value of each factor before and after tolvaptan treatment affected the completion of tolvaptan administration. A path analysis, based on structural equation modeling, was used to investigate the relationship between each factor, and their influence on the CDA was investigated. CDA was denoted as ‘1’ in cases where treatment could be completed within 7 days, and ‘0’ in cases where continuous administration was required. The path analysis was performed using the IBM SPSS AMOS software program (version 25, Amos Development Corporation, Meadville, PA, USA). We have previously described the method for creating the path model (15-17). In brief, the possible path model defined some hierarchical regression models among clinical factors and CDA. For every regression, the total variance in dependent variables was theorized to be affected either by independent variables that were included in the model or by extraneous variables. The structural equation model was tested, and P-values of < 0.05 were considered to indicate statistical significance.
Patient characteristics
The average duration of tolvaptan use was 3.8 ± 2.0 days in group A and 253 ± 318 days in group B, and the average dose of tolvaptan was 7.0 ± 2.1 mg/day in group A and 8.2± 3.9 mg/day in group B (NS). Table 1 shows the baseline characteristics of groups A and B.
|
Characteristics |
|
Overall |
Group A |
Group B |
P-values between groups |
|
|
|
|
Number (%) or Mean ± SD |
|
|||
|
Number |
|
102 |
35 |
67 |
|
|
|
Male gender |
|
56 (54.9) |
20 (57.1) |
36 (53.7) |
NS |
|
|
Age (years old) |
|
76.0±12.6 |
75.3±12.4 |
76.3±12.7 |
NS |
|
|
BUN (mg/dL) |
baseline |
30.4±13.8 |
30.1±16.7 |
30.6±12.2 |
NS |
|
|
Day 7 |
32.4±16.3 |
27.2±9.7* |
35.0±18.4 |
P<0.05 |
||
|
Cr (mg/dL) |
baseline |
1.49±0.87 |
1.54±0.96 |
1.47±0.83 |
NS |
|
|
Day 7 |
1.48±0.96 |
1.35±0.68 |
1.54±1.07 |
NS |
||
|
Na (mEq/L) |
baseline |
138.0±5.3 |
138.7±5.1 |
137.6±5.4 |
NS |
|
|
|
Day 7 |
139.1±4.0 |
138.4±4.4 |
139.4±3.7* |
NS |
|
|
K (mEq/L) |
baseline |
4.3±0.6 |
4.3±0.6 |
4.2±0.7 |
NS |
|
|
|
Day 7 |
4.4±0.5 |
4.4±0.6 |
4.3±0.5 |
NS |
|
|
BNP (pg/mL)
|
baseline |
869±762 |
1069±801 |
765±726 |
P<0.05 |
|
|
Day 7 |
527±935 |
524±766** |
529±1018* |
NS |
||
|
Hb (g/dL) |
baseline |
11.8±2.45 |
11.7±2.2 |
11.8±2.6 |
NS |
|
|
Day 7 |
12.2±2.6† |
12.8±2.6*** |
11.9±2.5 |
NS |
||
|
LVEF (%) |
|
43.6±15.9 |
38.6±12.6 |
46.0±16.8 |
P<0.05 |
|
|
BW(kg) |
baseline |
63.1±15.6 |
63.1±15.8 |
63.1±15.8 |
NS |
|
|
|
Day7 |
57.0±13.4 |
56.6±12.8 |
57.2±14.2 |
NS |
|
|
HR (beats/min) |
baseline |
96±26 |
100±26 |
94±26 |
NS |
|
|
|
Day7 |
76±15 |
76±15 |
76±15 |
NS |
|
|
UV(mL) |
baseline |
2742±930 |
2890±1159 |
2660±773 |
NS |
|
|
|
Day7 |
1383±312 |
1370±354 |
1389±289 |
NS |
|
|
sysBP (mmHg) |
baseline |
128±25 |
135±25 |
125±25 |
NS |
|
|
|
Day7 |
114±15 |
114±15* |
113±16 |
NS |
|
|
CTR (%) |
baseline |
63.0±6.0 |
63.0±6.3 |
63.0±5.8 |
NS |
|
|
|
Day7 |
57.5±5.9 |
56.3±6.5* |
58.1±5.5 |
NS |
|
|
|
Underlying cardiovascular disease number |
|
||||
|
|
|
|
||||
|
DCM |
|
12 (58.3) |
1(100.0) |
11 (54.5) |
P<0.05 |
|
|
IHD |
|
34 (70.6) |
14 (78.6) |
20 (65.0) |
NS |
|
|
Valvular diseases |
|
21 (33.3) |
6 (16.7) |
15 (40.0) |
NS |
|
|
Hypertension |
|
75 (73.5) |
24 (68.6) |
51 (76.1) |
NS |
|
|
Post PCI therapy |
|
34 (33.3) |
14 (40.0) |
20 (29.9) |
NS |
|
|
DM |
|
30 (29.4) |
8 (22.9) |
22 (32.8) |
NS |
|
|
Dyslipidemia |
|
33 (32.4) |
10 (28.6) |
23 (34.3) |
NS |
|
|
|
Baseline medication |
|
||||
|
ACE-Is / ARBs |
|
51 (50.0) |
20 (57.1) |
31 (46.3) |
NS |
|
|
Beta blockers |
|
49 (48.0) |
13 (37.1) |
36 (53.7) |
NS |
|
|
Loop diuretics |
|
71 (69.6) |
18 (51.4) |
53 (79.1) * |
P<0.01 |
|
|
MRAs |
|
44 (43.1) |
15 (42.9) |
29 (43.3) |
NS |
|
|
Digitalis |
|
12 (11.7) |
5 (14.3) |
7 (10.4) |
NS |
|
|
Statins |
|
34 (3.9) |
8 (22.9) |
26 (38.8) |
NS |
|
Table 1 Patient characteristics
BW, body weight; HR, heart rate; systBP, systolic blood pressure; CTR, Cardiothoracic ratio; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; Cr, creatinine; Na, serum sodium; K, serum potassium; Hb, Hemoglobin; LVEF, left ventricular ejection fraction; DCM, dilated cardiomyopathy; IHD, ischemic heart disease; PCI percutaneous coronary intervention therapy; DM, Type-2 diabetes mellitus; ACE-Is, angiotensin-converting enzyme inhibitors; and ARBs, angiotensin II type I-receptor blockers; MRAs, mineralocorticoid receptor antagonists.
*P < 0.05, **P < 0.01, ***P < 0.001; statistical significance in the comparison of clinical factors between baseline and day 7 after tolvaptan administration
P-values between groups denote the statistical significance of the comparison of the clinical factors between group A and B
There were no significant differences between the two groups with regard to sex, age, or the prevalence of basal diseases. The prevalence of diuretics use was significantly lower in group A than in group B (P< 0.01). The prevalence of other medications, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, and mineral corticoid receptor blockers did not differ between the two groups to a statistically significant extent.
Laboratory data and echocardiographic variables at admission and 7 days
The changes in the laboratory data at baseline and 7 days after the administration of tolvaptan are also provided in Table 1. In group A, the blood urea nitrogen (BUN) levels were significantly decreased (P < 0.05) and BNP level was significantly reduced (P < 0.01). In contrast, hemoglobin (Hb) levels were significantly increased (P < 0.001). In group B, the sodium (Na) level was significantly increased (P < 0.05) and the BNP level was significantly reduced (P < 0.05).
Significant differences were observed in the baseline BNP, and LVEF levels of groups A and B (BNP: P < 0.05, EF: P < 0.05). At baseline, loop diuretics were used significantly less frequently in group A than in group B (P < 0.01). There were no significant differences in the usage of other medications at baseline.
Statistical analysis of factors and associated changes
Tables 2 and 3 present the results of the univariate and multiple logistic regression analyses.
|
Dependent variable |
Logistic regression analysis |
||||||
|
CDA |
Univariate |
|
Multivariate |
||||
|
OR |
95% CI |
P values |
|
OR |
95% CI |
P-values |
|
|
Male gender |
- |
- |
- |
|
1.766 |
0.519, 6.011 |
NS |
|
Age |
0.994 |
0.962, 1.026 |
NS |
|
0.983 |
0.926, 1.044 |
NS |
|
BUN |
0.997 |
0.968, 1.028 |
NS |
|
0.967 |
0.909, 1.029 |
NS |
|
Cr |
1.101 |
0.694, 1.748 |
NS |
|
1.188 |
0.487, 2.898 |
NS |
|
Log BNP |
4.795 |
1.392, 16.513 |
NS |
|
1.527 |
0.219, 10.633 |
NS |
|
Hb |
0.994 |
0.840, 1.176 |
NS |
|
0.780 |
0.561, 1.086 |
NS |
|
LVEF |
0.969 |
0.941, 0.998 |
P<0.05 |
|
0.958 |
0.913, 1.005 |
NS |
Table 2 The results of the univariate and multiple logistic regression analyses to identify baseline clinical factors influencing the completion of tolvaptan administration
CDA, completion of tolvaptan (drug) administration; OR, odds ratio; CI, confidence interval; 95% CI, 95% confidence interval; R2, adjusted coefficient of determination; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; Cr, creatinine; Hb, hemoglobin, LVEF, left ventricular ejection fraction
These were perform
|
Dependent variable |
Logistic regression analysis |
||||||
|
CDA |
Univariate |
|
Multivariate |
||||
|
OR |
95% CI |
P values |
|
OR |
95% CI |
P-values |
|
|
Male gender |
- |
- |
- |
|
1.727 |
0.549, 5.434 |
NS |
|
Age |
0.994 |
0.962, 1.026 |
NS |
|
1.048 |
0.989, 1.111 |
NS |
|
BUN |
0.964 |
0.933, 0.995 |
NS |
|
0.948 |
0.894, 1.004 |
NS |
|
Cr |
0.778 |
0.462, 1.308 |
NS |
|
1.629 |
0.696, 3.811 |
NS |
|
Log BNP |
1.299 |
0.493, 3.421 |
NS |
|
0.667 |
0.164, 2.708 |
NS |
|
Hb |
1.142 |
0.968, 1.346 |
NS |
|
1.119 |
0.865, 1.448 |
NS |
|
LVEF |
0.999 |
0.999, 1.000 |
NS |
|
0.999 |
0.998, 1.000 |
NS |
Table 3 The results of the univariate and multiple logistic regression analyses to identify the day 7 clinical factors influencing the completion of tolvap tan administration
CDA, completion of tolvaptan (drug) administration; OR, odds ratio; CI, confidence interval. 95% CI, 95% confidence interval; R2, adjusted coefficient of determination; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; Cr, creatinine; Hb, hemoglobin, LVEF, left ventricular ejection fraction
Differences between groups A and B in the changes range (Δ) of each factor, before and after tolvaptan treatment
Table 4 shows the differences in the values of each factor between groups A and B before and after tolvaptan treatment. Group B showed significantly greater changes in BUN (ΔBUN), and creatinine (ΔCr) (group A vs. group B: ΔBUN, P < 0.05, and ΔCr, P < 0.001). On the other hand, the change in Hb (ΔHb) was significantly greater in group A than in group B (P < 0.001).
|
|
Group A (n=35) |
Group B (n=67) |
P-values |
|
ΔBUN (mg/dL) |
-2.89±18.6 |
4.46±14.4 |
P<0.05 |
|
ΔCr (mg/dL) |
-0.192±0.635 |
0.075±0.463 |
P<0.05 |
|
ΔNa (mg/dL) |
-0.314±7.13 |
1.77±4.42 |
NS |
|
ΔBNP (mg/dL) |
-545±858 |
-236±931 |
NS |
|
ΔHb (mg/dL) |
1.05±1.18 |
0.186±1.20 |
P<0.001 |
Table 4 Changes in each factor (Δ) after tolvaptan administration
Delta (Δ) represents the change in value [(the value on day 7) – (the value at baseline)]
BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; Cr, creatinine; Na, serum sodium; Hb, hemoglobin
P-values denote the statistical significance of the difference between group A and B
Structural equation modeling to search for factors associated with CDA
From the above results, it was inferred that BUN, Cr, and Hb are important for the CDA of tolvaptan. A further logical analysis is needed to identify factors associated with successful termination of tolvaptan. A path diagram of the structural equation modeling was devised and analyzed, as shown in Figure 2.
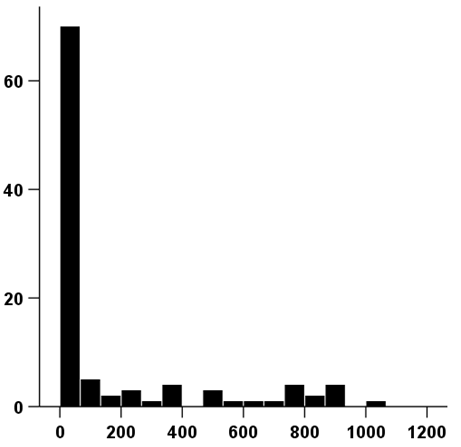
Figure 1 Chart showing the distribution of days of tolvaptan treatment.
The range of tolvaptan use was 1-1021 days, and the average period of use was 168 days.
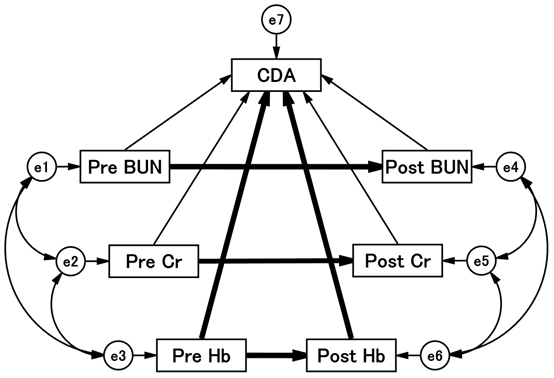
Figure 2 Path diagram with standardized regression coefficients for the independent variables against dependent variables.
The path shows standardized coefficients of the regressing independent variable for the dependent variable of the related path (BUN to CDA, BUN to CDA, Cr to CDA, Cr to CDA, Cr to CDA, Hb to CDA, Hb to CDA). Arrows are drawn before and after the administration of tolvaptan, and the direct or indirect effects of each factor on CDA are calculated. Detailed results are shown in Table 5. BUN, blood urea nitrogen; CDA, completion of tolvaptan administration; Cr, creatinine; Hb, hemoglobin.
|
Clinical Factor |
Regression coefficients |
P-values* |
|||||||||||||
|
Estimated |
Standardized effect |
||||||||||||||
|
Direct |
Indirect |
Total |
|||||||||||||
|
Pre-BUN |
→ |
Post-BUN |
0.589 |
0.483 |
0 |
0.483 |
< 0.001 |
||||||||
|
(R2=0.233) |
|
|
|
|
|
|
|
||||||||
|
Pre-Cr |
→ |
Post-Cr |
0.836 |
0.805 |
0 |
0.805 |
< 0.001 |
||||||||
|
(R2=0.647) |
|
|
|
|
|
|
|
||||||||
|
Pre-Hb |
→ |
Post-Hb |
0.909 |
0.875 |
0 |
0.875 |
< 0.001 |
||||||||
|
(R2=0.765) |
|
|
|
|
|
|
|
||||||||
|
Pre BUN |
→ |
CDA |
0.001 |
0.024 |
-0.103 |
-0.080 |
NS |
||||||||
|
Post BUN |
→ |
CDA |
-0.006 |
-0.214 |
0 |
-0.214 |
NS |
||||||||
|
Pre-Cr |
→ |
CDA |
0.141 |
0.254 |
-0.089 |
0.165 |
NS |
||||||||
|
Post-Cr |
→ |
CDA |
-0.059 |
-0.111 |
0 |
-0.111 |
NS |
||||||||
|
Pre-Hb |
→ |
CDA |
-0.107 |
-0.544 |
0.543 |
-0.001 |
P<0.01 |
||||||||
|
Post-Hb |
→ |
CDA |
0.118 |
0.621 |
0 |
0.621 |
P<0.001 |
||||||||
|
(R2=0.188) |
|
|
|
|
|
|
|
||||||||
|
C.C. |
P-values |
C.C. |
P-values |
C.C. |
P-values |
||||||||||
|
e1↔e2 |
0.593 |
< 0.001 |
e1↔e3 |
-0.307 |
P<0.01 |
e2↔e3 |
-0.279 |
P<0.01 |
|||||||
|
e4↔e5 |
0.686 |
< 0.001 |
e4↔e6 |
-0.135 |
NS |
e5↔e6 |
-0.191 |
NS |
|||||||
Table 5 Results of the path model
The results (direct, indirect, and total effects) of the theoretically proposed path model analysis to identify the clinical factors influencing each other (see Figure 2, path model)
*P-values: the marked P value denotes the evaluation value of the standardized direct effect coefficient. Cr, creatinine; Hb, hemoglobin; BUN, blood urea nitrogen; C.C., correlation coefficient; e, extraneous variable; R2, squared multiple correlations
Each was placed at baseline and day 7 of tolvaptan administration, and lines were drawn to the corresponding values. Lines were drawn from each of the factors, as all factors may affect CDA. The precise results of the path model are summarized in Table 5.
Figure 2 shows that the results of the theoretical path model analysis identified which of the clinical factors influenced each other. An exploratory factor analysis revealed that Hb levels played a causative role in the CDA. Hb levels were significantly associated with CDA before (standardized regression coefficient: -0.544, P < 0.01) and after (standardized regression coefficient: 0.621, P < 0.001) treatment. Low Hb levels before administration and high Hb levels after treatment were associated with CDA.
As shown in Tables 1 and 2, univariate and multivariate analysis was less clear as to which factors were associated with CDA. Perhaps it is associated with a complex conjugation problem between all factors before and after administration. However, studies of pre- and post-treatment value differences list BUN, Cr, and Hb as possible factors. We then used structural equation modeling to find out which of BUN, Cr, and Hb was most relevant to CDA. As a result, pre-treatment Hb and post-treatment Hb were associated with CDA. It was found that the lower the Hb before treatment, the higher the effectiveness of tolvaptan, and the higher the Hb after treatment, the higher the effectiveness of tolvaptan.
In particular, we believe that the relationship between pre-treatment Hb and CDA is an important finding. Acute decompensated heart failure with low Hb before treatment means that tolvaptan is likely to improve the congestion state. On the other hand, in the case of heart failure, which is suffering from acute decompensated heart failure even if Hb is high, the effect of tolvaptan cannot be expected so much.
After the EVEREST trial with tolvaptan, the clinical profile and prognostic value of anemia at admission and discharge in patients admitted with heart failure with reduced ejection fraction have been reported.18 As a result, among hospitalized HF patients with reduced ejection fraction, modest anemia at discharge but not baseline was associated with increased all-cause mortality and short-term cardiovascular mortality plus HF hospitalization. The result is not inconsistent with our result. This is because tolvaptan is thought to mean improving the prognosis of patients with anemia on admission. Tolvaptan may have reduced and offset the adverse effects of anemia on admission.
It is unclear how pretreatment low Hb is associated with the effects of tolvaptan. Simply put, heart failure can lead to a decrease in Hb due to increased water and dilution.19,20 Due to the strong diuretic effect of tolvaptan, a decrease in Hb before treatment may indicate the effectiveness of tolvaptan. However, if the effect of dilution is the same as BUN, it is unlikely that dilution alone can fully explain it.
In general, anemia is an independent prognostic factor in both acute and chronic heart failure.21,22 Therefore, it would be very interesting if there was any relationship between tolvaptan and the improvement of anemia. Of course, it is unknown at this time. However, interesting research on vasopressin and anemia has been done as follows.
The first report is that AVPR1B plays an important role in the regulation of red blood cell estimation in both human cells and mouse cells.23 Tolvaptan has the effect of selectively antagonizing AVPV2 only. On the other hand, as a secondary reaction, the effect of an increase in endogenous vasopressin on other V1A and V1B cannot be denied. In the next paper, chronic activation of vasopressin V2 receptor signaling has also been reported to reduce renal medulla oxygen levels in rats.24 Vasopressin is thought to promote the progression of renal anemia; and so tolvaptan may be able to block it. In addition, other papers strongly suggested that hypoxia-inducible factor (HIF) is involved in vasopressin and hypoxia.25,26 In the future, it will be necessary to closely investigate the direct or indirect relationship between tolvaptan and the improvement of anemia in heart failure and renal failure. Perhaps the combined use of HIF-PH inhibitors and tolvaptan may be expected. This time, the underlying disorder of anemia has not been examined. Since it was a retrospective study, there was a shortage of research data. For example, measurements of serum iron, ferritin, total and unsaturated iron binding capacity, transferrin saturation, hepcidin, etc. may allow detailed examination of iron utilization. We would definitely like to go on to the next research.
Current research has some limitations. 1) The survey was conducted in a single facility with a small sample size. 2) Second, the determination of tolvaptan efficacy is at the discretion of the attending physician, and the possibility of biasing the results cannot be ruled out. However, the effectiveness of tolvaptan was determined based on chest radiographs and changes in urine output, so I think the bias is minimal. 3) Due to the use of various drugs in addition to loop diuretics and tolvaptan, the potential involvement of these drugs cannot be ignored. 4) The cause and severity of pretreatment anemia are unknown. 5) Path analysis does not represent cause and effect. It's just a relationship, and we may need to devise other path diagrams in the future.
In acute decompensated heart failure, decreased Hb levels at admission were associated with the efficacy of tolvaptan treatment after 7 days. Hb reduction may infer the effectiveness of tolvaptan before treatment. In that case, active use of tolvaptan is desired.
Declared none.
The authors declare no conflicts of interest directly related to this study.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.

©2022 Shibata, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.