Journal of
eISSN: 2373-4396


Research Article Volume 16 Issue 3
Department of Cardiology, Zagazig University Hospital, Egypt
Correspondence: Ragab A Mahfouz, Professor of Cardiology, Zagazig University, faculty of medicine; Egypt, Tel 20016427671, Fax 20552357770
Received: May 24, 2023 | Published: July 5, 2023
Citation: Mahfouz RA, Amin M, Arab M. Relation of exercise intolerance to microvascular dysfunction in COVID-19 recovered patients after six months of recovery. J Cardiol Curr Res. 2023;16(3):80-85. DOI: 10.15406/jccr.2023.16.00583
Aims: Our aim was to explore the relation between coronary microvascular function, as assessed with transthoracic echocardiographic Coronary Flow Reserve (CFR) and exercise tolerance in COVID-19 recovered patients after 6 months of recovery.
Methods: 79 patients with COVID-19 with a mean age (51±12) were recruited 6 months after recovery. All patients underwent transthoracic echocardiographic evaluation of coronary flow reserve (CFR). Furthermore, they underwent self-limited exercise tolerance test (ETT).
Results: Based on the metabolic equivalents (METS), participates were stratified to a group with exercise intolerance with METs ≤8 and another group with good exercise tolerance with METs >8. Patients with exercise intolerance had significantly lower CFR (1.8 ± 0.3 vs. 3.1 ± 0.5; P <0.001), Patients with reduced exercise tolerance (METs ≤8) had higher E/e' ratio and left atrial volume index when, compared to subject with METs ≤8 (p< 0.01). Furthermore, brain natriuretic peptide, troponin-I, hs-C reactive protein, lactic dehydrogenase during the acuteness period were considerably elevated in recovered patients with METs <8. Moreover, CFR had significant inverse correlations with E/e' (r = −0.45; P < 0.001). At multivariate analysis CFR appeared to be a sponge independent predictor of reduced exercise tolerance (METs<8) in COVID-19 recovered patients (p<0.001)
Conclusions: The current research revealed a significant association between coronary microvascular dysfunction and reduced exercise tolerance and diastolic dysfunction in patients with COVID-19 six months after recovery. Fore that reason, we suggested that microvascular dysfunction is a possible mechanism of exercise intolerance after COVID-19 recovery.
Keywords: COVID-19, coronary flow, exercise tolerance, angiotensin-converting enzyme 2
ACE-2, angiotensin-converting enzyme 2; PASC, Post-Acute Sequelae of SARS-CoV-2 Infection; RT-PCR, reverse transcription-polymerase chain reaction; CFR, coronary flow reserve; LAD, left anterior descending
Various direct or indirect cardiovascular adverse events are related to COVID-19 infection, such as myocarditis, myocardial injury, arrhythmia, myocardial dysfunction, and venous thromboembolism.1,2 Myocardial injury, ischemia or necrosis is associated with diastolic or systolic dysfunction that results in higher mortality risk in those patients.3 Reports showed that, nearly, twenty to thirty percent of COVID-19 patients, who were admitted to hospitals, have cardiac affection, as evidenced by high cardiac enzymes, which was linked with unfavorable prognosis.4–6 The underlying mechanisms of many organ involvement in COVID-19 patients are not clearly recognized. However, of a proposed mechanism is the angiotensin-converting enzyme 2 (ACE-2), which is widely uttered in many body systems, as the respiratory and cardiovascular systems, vascular endothelium, kidneys as well as gastro-intestinal tract.7 Moreover, host immune system dysregulation with augmented cytokine release and increased inflammatory reactions.8 In addition, the proinflammatory status could lead to microvascular dysfunction and disseminate intravascular coagulation.9
Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) is defined as a gathering of new, recurring, or insistent clinical problems presented by subjects 4 or more weeks following SARS-CoV-2 infection. It is a wide group of cardiovascular situations that include, but are not limited to, myocarditis and myocardial involvement, pericarditis, new or worsening myocardial ischemia, microvascular affections, nonischemic cardiomyopathy, thromboembolism, and arrhythmia.10
Our rationale was to explore the underlining pathophysiologic justification of reduced exercise tolerance in post-COVID-19 patients. We supposed that, the exaggerated inflammatory response of COVID-19 infection may lead to coronary microvascular dysfunction. For that, we amid to explore the relation between reduced exercise tolerance and microvascular dysfunction in recovered COVID-19 patients
Seventy nine consecutive subjects recovering from COVID-19 after confirmed diagnosis with chest computed tomography, laboratory information and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) of oropharyngeal swabs. We enrolled subjects after 6 months of recovery, who presented with unexplained dyspnea and or anginal chest pain.
Inclusion criteria
Patients with atrioventricular conduction disturbance, non-sinus rhythm on ECG, ventricular extra-systoles or any unstable cardiac rhythm, a permanent cardiac pacemaker, valvular heart disease, LVEF<50% with signs and symptoms of heart failure or LVEF ≥ 50% with signs and symptoms and elevated BNP, chronic rheumatic heart disease, unstable angina or acute coronary syndrome. Moreover we excluded patients with acute pulmonary embolism, uncontrollable arterial hypertension, acute pericarditis or myocarditis; active hepatitis or severe chronic liver diseases, endocarditis; intracerebral hemorrhage, transient ischemic attack and stroke in past medical history, chronic kidney disease, significant thyroid dysfunction, active autoimmune problems or neoplasms and patients taking, immunosuppressants or glucocorticosteroids.
This study was carried out according to the principles of Helsinki Declaration. All participates gave written informed written informed consent.
Exercise test
Participates carried out symptom‐limited treadmill exercise testing based on the modified Bruce protocol. Development of symptoms, development of sever hypertension or hypotension, distressing rhythm, considerable ST‐segment changes or reaching maximal predicted heart rate were the causes of test termination.
Exercise capacity was the workload of the subjects defined as units of METABOLIC EQUIVALENTS (METs). Good exercise capacity was defined as METs ≥8 METs, however, reduced exercise capacity was defined as METs less than 8.11
Echocardiographic examination
Transthoracic Echocardiographic assessment was performed using VIVID 5S (GE Healthcare Systems, Horten, Norway) with a 2.0–3.5-MHz transducer 6months after clinical recovery in patients with COVID-19. Echocardiographic and Doppler evaluations were performed based on the guidelines of the AMERICAN SOCIETY OF ECHOCARDIOGRAPHY.12
Modified Simpson’s method was used to calculate left ventricular ejection fraction (LVEF%). In addition, left atrial volume index (LAVI ml/m2), E and A wave velocities of trans- mitral flow. Tissue Doppler imaging with to obtain mitral annular velocities e' and a'. Then E/e' ratio was measured a marker of LV filling pressure.
Coronary flow reserve (CFR) assessment (Figure 1)
With a high frequency transducer (5 to 7 MHz) and the guidance of color Doppler flow mapping, the distal portion of the left anterior descending artery (LAD) was imaged at modified apical 4 chamber view. A 2.5mm sample volume was placed on the left anterior descending coronary artery color-flow signal to obtain coronary flow spectral tracing. Doppler signal was obtained at rest and the peak diastolic velocity was recorded. Then, IV adenosine was administered (0.14mg/kg/min) to record the hyperemic peak diastolic velocity. The average of three peak diastolic velocities was obtained of both at baseline and hyperemia. After that, we calculate the coronary flow reserve as the ratio of peak hyperemic diastolic velocity over baseline peak diastolic velocity. Coronary flow reserve less than 2.5 was considered a microvascular dysfunction.13
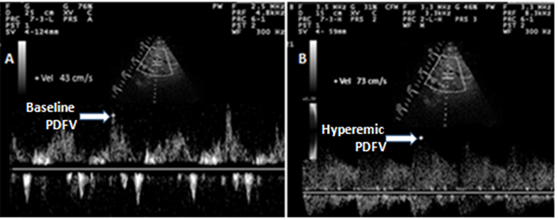
Figure 1 Spectral Doppler image with of transthoracic echocardiography of coronary flow.
A- Baseline peak Diastolic Coronary Flow Velocity (Baseline PDV).
B-Hyperemic peak Diastolic Coronary Flow Velocity (Hyperemic PDV).
To determine the reproducibility of CFR, a total of 20 randomly selected evaluations were examined twice by one investigator at a 1-week interval and once by another investigator.
Statistical analysis
Study information was presented as (mean ± SD). for continuous variables and as percentages for categorical variables. Comparison between parametric values were evaluated with the use of independent sample t-test, while, the nonparametric values were compared with Mann–Whitney tests. Correlations between parametric variables were analyzed with Pearson’s test, whilst, non-parametric variables were tested with Spearman’s test. To evaluate the predictors of reduced exercise tolerance in COVID-19 recovered patients, Logistic regression analysis was used. Multivariate logistic regression analysis included univariate variables with p<0.1. All data were analyzed with the use of SPSS v. 22.0 for window (Chicago, USA).
Seventy-nine COVID-19 recovered subjects were stratified in 2 groups according to the metabolic equivalents (METs) with self-limited exercise ECG. Group with reduced exercise tolerance, included 35 (44.3%) patients with METs <8 and the other group with good exercise tolerance and included 44 (55.7) patients with METs ≥ 8. The demographic characteristics were comparable between both groups (Table 1).
|
Variable |
COVID-19 recovered patients |
P value |
|
|
|
<8 METs, n(35) 44.3% |
≥ 8 METs, n(44)55.7 |
|
|
Age, y |
52± 11 |
50 ±10 |
0.11 |
|
Men, n (%) |
21(60) |
28(63.6) |
0.49 |
|
Body mass index (kg/m2) |
26± 3.2 |
25.9 ± 3.0 |
0.15 |
|
Smokers, n (%) |
13(38.2) |
18(40.9) |
0.13 |
|
Hypertension, n (%) |
11(31.4) |
15(34.1) |
0.41 |
|
Diabetes, n (%) |
13(37.1) |
17(38.6) |
0.39 |
|
Systolic blood pressure, mmHg |
129±13 |
127±15 |
0.21 |
|
Diastolic blood pressure, mmHg |
79±8 |
80±8 |
0.42 |
|
Heart rate (bpm) |
73± 10 |
72 ± 9 |
0.12 |
|
Blood glucose (mg/dL) |
119 ± 8 |
121 ± 11 |
0.61 |
|
Total cholesterol (mg/dL) |
201 ± 33 |
198± 25 |
0.25 |
|
Low density lipoprotein (mg/dL) |
128±21 |
125±20 |
0.52 |
|
High density lipoprotein (mg/dL) |
41±8 |
45±7 |
0.14 |
|
Triglycerides (mg/dL) |
142±38 |
147±43 |
0.33 |
|
Creatinine (mgl/dL) |
1.3 ± 0.3 |
1.1 ± 0.3 |
0.13 |
|
Laboratory data during acuteness period |
|
|
|
|
D-dimer, mg/L |
0.9±0.13 |
0.8± 0.8 |
0.67 |
|
Brain natriuretic peptide (pg/mL) |
217± 41 |
63 ± 11 |
<0.001 |
|
hs-C reactive protein, mg/dL |
6.9 ± 1.5 |
3.4 ± 0.5 |
<0.001 |
|
hs- troponin-T, pg/dL |
5.1 ± 0.4 |
2.3 ± 0.3 |
<0.001 |
|
Lactic dehydrogenase, IU/L |
513±125 |
309.4±105 |
<0.03 |
|
Ferritin, ng/mL |
518 ±91 |
392± 46 |
0.19 |
|
Exercise test |
|
|
|
|
Exercise duration, min |
6.6±1.5 |
10.2±2.9 |
<0.001 |
|
Metabolic equivalents (METs) |
5.0±1.6 |
10.1±2.1 |
<0.001 |
|
Duke treadmill score |
5.1±3.9 |
6.7±4.3 |
<0.001 |
Table 1 Comparison between patients with COVID-19 based on their metabolic equivalents METs <8or ≥ 8
Compared with COVID-19 recovered patients with METs≥ 8, those with METs <8 had a significantly higher hs-troponin T (5.1 ± 0.4versus 2.3 ± 0.3 pg/dL, p<0.001), lactic dehydrogenase (513±125 versus 309.4±105 IU/L, p<0.03), brain natriuretic peptide (217± 41versus 63 ± 11 pg/mL, p<0.001) and hs-CRP (6.9 ± 1.5versus 3.4 ± 0. mg/dL, p<0.001) during their acuteness period.
With respect to CFR and echo-Doppler study (Table 2), subjects with reduced exercise tolerance had significantly reduced CFR compared in comparison to patients with good exercise capacity (1.8±0.3 versus3.1±0.5, p<0.001), figure-1. Furthermore, E/e' ratio (p<0.01), LAVI (p<0.01) were considerably increased in patients with reduced exercise tolerance.
|
Variable |
COVID-19 recovered patients |
P value |
|
|
|
<8 METs, n (35) |
≥8 METs, n (44) |
|
|
LA volume index (mL/m2) |
38.5±5.8 |
25.4±3.9 |
<0.01 |
|
LV EF% (%) |
64.5±6.1 |
66.9±5.7 |
0.13 |
|
E/A ratio |
1.09±0.3 |
1.2±0.3 |
0.11 |
|
E/e′ ratio |
13.7±3.5 |
9.1±2.5 |
<0.01 |
|
Resting DCFV |
26±5 |
24±4 |
0.37 |
|
Hyperemic DCFV |
45±10 |
75±16 |
<0.01 |
|
Coronary flow reserve |
1.8±0.3 |
3.1±0.5 |
<0.001 |
Table 2 Echocardiographic parameters in COVID-19 recovered patients based on METs <8 or ≥ 8 METs
LA: Left atrium, LV: left ventricle, LVEF: left ventricular ejection fraction, DCFV: diastolic coronary flow velocity.
Correlation analysis (table-3) shows that METs was significantly correlated with CFR (r=0.48, p<0.001), and negatively associated with E/e' ratio(r=−0.28, p<0.05) and LAVI (r=−0.31, p<0.05), hs-CRP (r=-0.39, p<0.001), hs-troponin T (−0.25, p<0.05) and BNP (r=−0.31, p<0.03). Likewise, the CFR has a significant correlation with E/e' ratio (r = −0.45; P < 0.001) (Figure 2).
|
|
r |
P value |
|
Age, year |
−0.20 |
0.09 |
|
Brain natriuretic peptide |
−0.31 |
< 0.03 |
|
hs- troponin I |
−0.25 |
<0.05 |
|
hs C-reactive protein mg/L |
−0.39 |
<0.003 |
|
Left atrial volume index, ml/m2 |
−0.31 |
< 0.05 |
|
E/e' |
−0.28 |
< 0.05 |
|
Coronary flow reserve |
0.48 |
< 0.001 |
Table 3 Correlation analysis between metabolic equivalents and univariate variables in COVID-19 patients at 6 months of recovery
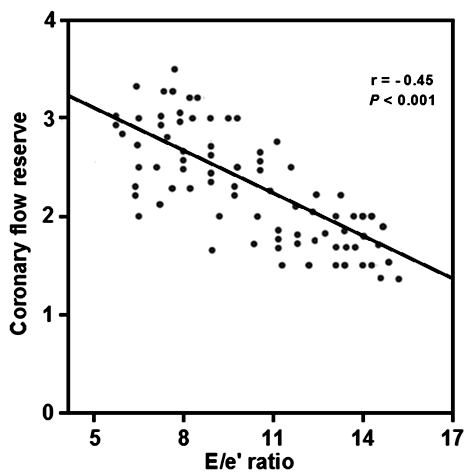
Figure 2 Correlation between E/e' ratio and Coronary Flow Reserve of COVID-19 recovered patients coronary flow reserve.
At Univariate analysis, CFR (<0.001), E/e' (<0.03), LAVI (p<0.01), hs-CRP (p<0.003), BNP (p<0.01) and hs-troponin I (p<0.01) were independently associated with METs <8. Multivariate analysis showed that coronary flow reserve was the strongest (p<0.001) independent predictor of decreased exercise capacity (METs) in COVID-19 recovered subjects. The hs-CRP during acuteness period was also independently associated reduced exercise tolerance in those patients (p<0.05) (Table 4).
|
|
Variable |
Univariate |
Multivariate |
||
|
|
OR (95%C.I) |
p value |
OR (95%C.I) |
p value |
|
|
Brain natriuretic peptide |
1.89 (1.02–3.11) |
<0.01 |
1.35 (1.06-1.73) |
0.13 |
|
|
hs-C reactive protein mg/L |
1.95 (1.42-3.15) |
<0.003 |
1.75 (1.33-2.65) |
<0.05 |
|
|
hs-Troponon I |
1.81 (1.19-2.53) |
<0.01 |
0.98(0.95-1.13) |
0.19 |
|
|
Left atrial volume index |
2.25 (1.15–3.27) |
<0.01 |
1.62(1.01-2.52) |
0.09 |
|
|
E/e' ratio |
1.59 (1.02-2.25) |
<0.03 |
1.42 (0.98-1.83) |
0.13 |
|
|
Coronary flow reserve |
3.17 (1.20-5.79) |
<0.001 |
2.65 (1.19-4.85) |
<0.001 |
|
Table 4 Univariate and multivariate predictors for reduced exercise capacity in COVID-19 patients at 6 months of recovery
The current research revealed that; 1) 44.3% of patients with COVID-19 have reduced exercise capacity 6 months after recovery; 2) reduced exercise capacity is significantly linked to microvascular and diastolic dysfunction; 3) microvascular dysfunction is independently associated with exercise intolerance and significantly linked to diastolic dysfunction in those patients.
Earlier data showed that impaired microvascular function in patients with acute viral infection were observed during the influenza A (H1N1) pandemic. Salgado et al. reported that microvascular function was severely impaired in subjects with critical acute lung injury.14
It has been supposed that microemboli and/or microvascular may be an important pathogenic mechanism of acute coronary injury that might occur in COVID-19 patients. Preclinical investigations on microembolization confirmed vascular constriction, enhanced inflammatory reaction, and significant augmentation of the levels of tumor necrosis factor α.15
Reports suggested that microvascular dysfunction is a potential pathogenic mechanism underlying cardiac dysfunction in COVID-19-positive patients. The enhanced systemic inflammatory reaction in COVID-19-positive patients and the and the relied endothelial impairment found to be a contributing factor microvascukar dysfunction. Furthermore, COVID-19 could have a direct action on micro-vasculatures, seeing that ACE-2 receptor is expressed as well on the surface of vascular endothelium.16 Chen et al.15 showed that cardiac peri-cytes have an elevated expression of ACE-2, therefore, supporting the suggestion that peri-cyte injury as a result of viral infections, might lead to capillary endothelium lining cellular injury and microvasculature dysfunction.17
Studies revealed that, acute cardiac injury was associated with COVID-19 acute disease phase only. They supposed that endothelial dysfunction and autoimmune reactions lead to massive proinflammatory cytokine release and prothrombotic scene. Noticeably, such pathogenic reactions associated with acute disease phase might result in long-lasting coronary microvascular dysfunction. These changes are usually augmenting myocardial and endothelial damage, with a subsequent continued coronary microvascular dysfunction. Furthermore, based on the disturbed autoimmune reactions and variation of coronary blood flow in reaction to different stimuli, it was hypothesized that microvascular dysfunction is present in post-COVID-19 subjects still complaining exertional dyspnea and or angina chest pain.18–20
Amusingly, up to 90% of subjects with COVID-19 had reduced vascular density, entirely limited to microvasculature. Likewise, numerous serum indicators of impaired endothelium were augmented and linked with disease severity in patients with COVID-19. Endothelial dysfunction); myocardial coronary arterioles and capillaries y and preceding microthrombi due to the prothrombotic vascular milieu were speculated to be the major players for the existence of microvascualr dysfunction in post-COVID-19 subject.21,22
We found that patients with reduced exercise tolerance had higher BNP, hs-troponin-I and hs-CRP than patients with good exercise tolerance. The higher laboratory markers of those patients were significantly associated with reduced CFR.
Inflammation, usually, stimulates endothelium resulted in overexpression of adhesion molecules, motivated by pro-inflammatory cytokines and chemokines include interleukine-1β, tumor necrosis factor-α and CRP. (23) Thromboembolization behavior, reflecting a close relation with augmented pro-inflammatory cytokines, may result in impaired vascular endothelium and thromboembolization. This vicious circle concerning cytokines augmentation and injury of vascular endothelium emerged to be an important contributor of various organs dysfunction in subjects with severe COVID-19. Therefore, therapeutic approaches to alleviate organ inflammation and injuries have been planned. In addition, 24,25 Randomised Evaluation of Covid-19 Therapy (RECOVERY) trial demonstrated favorable impact of corticosteroids for subjects with COVID-19 who on need for respiratory support.26 Importantly, corticosteroids, along through mineralocorticoid receptors, interact with Renin-angiotensin in COVID-19 patients.27
The inflammatory burden of COVID-19 usually interacts with epicardial fat and might provide a plausible link with microvascular dysfunction. It was observed that, significant percentage of COVID-19 recovered patients had left ventricular diastolic dysfunction. In addition, all biological markers of cardiac injury heart damage and inflammation were significantly elevated.28
Puntmann et al.29 demonstrated that 78%COVED-19- recovered patients' increased left ventricular mass index and impaired both ventricles functions, as assessed by MRI, regardless basic cardiac situation and COVID-19-severety score. Furthermore, about 60% of COVID-19 study subjects had significant increase in cardiac enzymes. Moreover, myocardial inflammatory reaction, edematous and fibrotic changes of the myocardium were observed as detected by atypical native T1 and T2 results.
While the underlying pathognomic mechanisms of COVID-19 related cardiac disease are not clear, initial reports imply that regional and all systems inflammatory reactions take part in the initiation of new myocardial dysfunction or augmenting an existing heart disease.30
Univariate analysis revealed that left atrial volume index, left ventricular filling, and coronary flow reserve were independent predictors of reduced exercise tolerance in recovered COVID-19 patients. On the other hand, at multivariate analysis, CFR appeared to be the only independent predictor of reduced exercise tolerance in recovered COVID-19 patients.
Previous investigators reported that diastolic dysfunction was associated with reduced VO2 peak in patients with chronic heart failure.31,32 In a previous study, we found that, as the CFR more reduced, the E/e' more increased.33 It was demonstrated that, severely impaired diastolic function is usually correlated with lower cardiac preload reserve. Furthermore, decreased vasodilatation reaction to exercise is related to decreased diastolic functional reserve.34
In their study, Borlaug et al.35 demonstrated that increased arterial stiffness was significantly associated with reduced vasodilatation response to exercise. This relation account for blunted diastolic functional reserve, which may be related to microvascular dysfunction.
These arguments implicate microvascular dysfunction as underlying mechanistic factor for diastolic dysfunction and reduced exercise tolerance in recovered COVID-19 patients and importantly implicate ongoing inflammation post-COVID-19 infection even after.
The clinical relevant of the current study is that post-COVID-19 patients, still at higher for cardiovascular adverse outcomes in short and long term follow-up, spite of resolved acute disease phase reactions. For that reason, continuous monitoring of those patients is an important issue for cardiovascular health.
First, small sample size. Second, single center study. Third, younger recovered patients with COVID-19 were not recruited for the study.
We found a significant association between coronary microvascular dysfunction as evaluated by CFR and reduced exercise tolerance and diastolic dysfunction in COVID-19-recovered patients, six months after recovery. For that reason, we suggested that microvascular dysfunction is a possible mechanism of exercise intolerance after COVID-19 recovery.
None.
Author declare there no conflicts of interest.
None.

©2023 Mahfouz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.