Journal of
eISSN: 2373-4396


Case Report Volume 16 Issue 3
Interventional and Structural Cardiology, Heart Failure Specialist, Germany
Correspondence: Dr. Ashish K. Mohapatra, Senior Consultant, Interventional and Structural Cardiology, Heart Failure Specialist, Germany, Tel , Tel +49-17647840414
Received: March 15, 2023 | Published: June 5, 2023
Citation: Mohapatra AK, James C. Rejuvenating a failing heart: A Case report. J Cardiol Curr Res. 2023;16(3):66-69. DOI: 10.15406/jccr.2023.16.00580
Heart Failure (HF) is a complicated, fatal medical condition that poses a severe threat to human life. It is a complex ailment that can lead to death and is associated with high healthcare costs, significant morbidity and mortality rates, and a reduction in functional capacity and quality of life. Recent data analysis reveals that approximately 64million people worldwide are affected by HF.1 Patients with HFrEF can benefit from cardiac contractility modulation (CCM), a revolutionary device-based treatment. In patients with HFrEF, CCM treatment has been associated with an improvement in exercise tolerance, an improvement in quality of life, a decrease in HF hospitalizations, and a reversal remodeling of the left ventricle. In this instance, we describe the therapeutic benefit of CCM in an elderly individual with advanced HFrEF triggered by ischemic dilated cardiomyopathy who frequently required hospitalization for heart failure-related issues and had a low quality of life despite receiving the best possible guideline-directed medical therapy (GDMT).
Keywords: Heart failure, device-based therapy, cardiac contractility modulation, device-based therapy, CRT-Nonresponder, ischemic cardiomyopathy, hospitalization, quality of life
HF, heart failure; CCM, cardiac contractility modulation; GDMT, guideline-directed medical therapy; HFrEF, HF with reduced ejection fraction; ICD, implantable cardioverter–defibrillator; CRT, cardiac resynchronization therapy; EF, ejection fraction; LCX, left circumflex artery; LAD, left anterior descending artery; ARNI, angiotensin-rezeptor-neprilysin-inhibitor; IPG, implanted pulse generator
Heart failure is a complicated clinical illness characterized by the heart's impaired capacity to pump and/or fill with blood.2,3 HF can be described physiologically as either insufficient cardiac output to fulfill metabolic needs or enough cardiac output related to compensatory neurohormonal activation (usually shown as increased left ventricular filling pressure).2 According to the ejection fraction, natriuretic peptide levels, and the presence of structural heart disease and diastolic dysfunction, HF has recently been classified into three subtypes: HF with reduced ejection fraction (HFrEF), and HF with mid-range ejection fraction (HFmrEF), HF with preserved ejection fraction (HFpEF).4 Despite breakthroughs in pharmacologic and nonpharmacologic treatment, many patients with heart failure and reduced ejection fraction (HFrEF) continue to have a clinical history marked by repeated illness readmission, decreased quality of life, and a poor prognosis.5,6
As this is a case report of one HFrEF, let us focus on the management of HFrEF only. In patients with HFrEF, optimized guideline-directed medical therapy (GDMT) and device therapy according to the patient’s phenotype have been suggested.7 In patients with ischemic cardiomyopathy and persistence of a left ventricular ejection fraction (LVEF) ≤35% despite optimized Guideline directed medical therapy(GDMT) for a minimum of three months, an implantable cardioverter–defibrillator (ICD) is recommended as primary prophylaxis to prevent arrhythmic sudden cardiac death (class I -A). But when a patient presents with HFrEF (LVEF ≤ 35%) with an electrocardiogram (ECG) of left bundle-branch block-type morphology with QRS ≥ 150 ms despite optimized GDMT for three months, one cardiac resynchronization therapy (CRT) device is recommended (class I-A). However, only one-third of the HFrEF Patient Population meets these criteria and approximately 30% of the guideline-directed CRT recipients do not experience significant benefits and quality of life, defined as clinically “non-responders”.8
Randomized clinical trials and a clinical registry have shown that cardiac contractility modulation (CCM) therapy represents an emergent therapeutic option in improving exercise capacity and quality of life, alleviating HF-related symptoms, and reducing HF-related hospitalizations in patients with symptomatic HF despite GDMT, LVEFs between 25% and 45%, who are not eligible for CRT9,10 as well as CRT-Nonresponders(CRT-NR).11–13
CCM is an emergent therapy, it employs standard pacing electrodes to deliver non-excitatory high-voltage biphasic impulses (~7.5 V/20ms) duration during the absolute refractory period of the action potential of cardiac myocytes (Figure 1).13,22
In this case report, we demonstrate the therapeutic benefit of this innovative HF device-based treatment in an older patient with dilated cardiomyopathy due to ischemic etiology and CRT-NR.
In June of 2012, a 64-year-old gentleman sought medical attention at the cardiac emergency department (ED) due to breathing difficulties and swelling in his feet that had been persistent for two weeks. He was diagnosed case of diabetes mellitus Type-2 in 2009 and since then under oral antidiabetic Drugs(Metformin and Glimepiride). An initial 12-lead ECG in ED was done, which was sinus rhythm with a heart rate of 94/min without any ST-T Changes.
The laboratory tests indicated that the Hemoglobin level was 13.2mg/dl, kidney function and electrolyte levels were normal, the Transaminases were slightly elevated, and the Troponin levels were high with a value of 24pg/ml (ref.<14 pg/ml) without any significant changes in the second test (29pg/ml). D-Dimer levels were normal, but the NTPro BNP levels were significantly elevated with a value of 6800 (ref <125pg/ml). The Echocardiography revealed normal LV Function with an ejection fraction (EF) of 60% and diastolic dysfunction grade I. During the hospital treatment, the patient's elevated blood pressure was noted through 24-hour blood pressure measurement and was diagnosed as Hypertension Grade II. The patient received diuretics and antihypertensive treatment (ACE-Inhibitor and Calcium Channel blocker). The patient was discharged after a recompensation.
In April 2015, he suffered from acute myocardial infarction( anterior wall-ST-Elevated Myocardial Infarction ). The Patient underwent an emergency angiogram, which revealed a two-vessels coronary artery disease with occluded left anterior descending artery (LAD) and 90% stenosis of the Left circumflex artery(LCX). he underwent a primary PTCA of LAD and staged PTCA of LCX before discharge. He was discharged with dual- antiplatelet therapy, Betablocker, and antihypertensive therapy. At the time of discharge, the echocardiography was with normal left ventricular function with grade I diastolic dysfunction. After uneventful 2 years, again the patient presented in the emergency department in 2017 with recurrent palpitation and syncope. He was diagnosed with paroxysmal atrial fibrillation 6 months before and under treatment with an optimized beta-blocker and anticoagulation. A further evaluation with 48 hours of Holter Monitoring revealed a tachy-brady Syndrome (Sick-sinus syndrome). As per the Guidelines, he underwent one dual-chamber pacemaker(DDD) implantation.
In 2018 summer, he was admitted to the cardiology department with complaints of dyspnea, and bilateral pedal edema. The patient was treated with intensive diuretic therapy. The Pacemaker interrogation revealed 92% ventricular stimulation, 80% atrial stimulation, and 5 phases of tachyarrhythmia absolute in the last 6 months period. Hence it is evident for one binodal disease. A 12-lead ECG showed pacemaker rhythm and left bundle branch block with QRS 168ms. Echocardiographically severe left ventricular dysfunction with EF 30% and dyssynchrony. In the laboratory investigation highly elevated Nt Pro BNP(8700 pg/ml), mildly reduced kidney function, and iron deficit. Radiologically pulmonary congestion. After successful decongestive treatment, a guideline-directed medical therapy(GDMT) was initiated with Angiotensin-Rezeptor-Neprilysin-Inhibitor(ARNI), Mineralocorticoid receptor antagonist(MRA), Beta blocker(patient had already) and SGLT-2 Inhibitor (Empagliflozin, started by the treating diabetologist since 2017).
In 2019, again he was admitted with symptoms of heart failure and pulmonary edema. Laboratory investigation showed deranged kidney function and elevated NT Pro BNP (21000, ref <125 pg/ml). Electrocardiogram revealed LBBB with bright QRS (182ms), and echocardiography revealed LEF of 30% and dyssynchrony, moderate Mitral regurgitation, and moderate Tricuspidal regurgitation. He was treated with intensive decongestive therapy and a ventilator, after a few days the patient was successfully weaned off from the ventilator. After an elaborate discussion with the patient, the dual-chamber Pacemaker was upgraded to CRT-D Device.
In 2020, the same patient was admitted to the emergency department with pleural effusion and pedal edema, and breathing difficulty for several days. Echocardiographically severe left ventricular dysfunction (LVEF 30%)(Figure 2), the blood investigation showed further deranged kidney function, and high Nt Pro BNP (14000 pg/ml). Vital parameters like Blood pressure of 92/60mm Hg, Herat rate of 70/min, and CRT Interrogation showed 99% biventricular stimulation. Considering the whole clinical parameter and CRT Function, it was diagnosed as CRT- Nonresponder. Because of low Blood pressure and deranged kidney function further optimization of guideline-directed medical therapy was not possible.
As the patient suffered from chronic heart failure and recurrent heart failure hospitalization and no scope for further optimization of the guideline-directed medical therapy and CRT-Nonresponse, we decided to implant the novel device called Cardiac contractility modulation (CCM) therapy. After getting the informed consent signed, one optimizer Smart device(Impulse Dynamics) for enhancing cardiac function was implanted. As mentioned earlier in the case report, there was a deranged kidney function, we have taken proper precautions and no contrast was injected. Postoperative CCM therapy (Figure-3)was initiated for 6 hours to observe the tolerance of the patient and to note any discomfort during the CCM therapy. The next day without registration of any discomfort and good tolerance, the CCM-Therapy was escalated to 8hours a day.
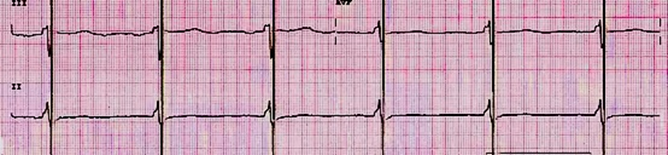
Figure 3 Electrocardiogram with CCM, Artifacts of CCM Stimulation in the absolute refractory period.
At 3months follow-up, there was a significant clinical improvement, no pedal edema, breathlessness NYHA II(previously NYHA III-IV), significant improvement in Quality of Life, and slight improvement of kidney function. The CCM Therapy was escalated to 10 hours a day. The patient could not do the 6 months follow-up, as he was out of the country for holidays with family after 8 years. At 8 months follow-up, further improvement in Quality of life, NT Pro BNP was 300 pg/ml, kidney function was normalized and echocardiographically LVEF 44%(Figure-4), mild mitral regurgitation and mild tricuspidal regurgitation, clinically no pedal edema, no pleural effusion. After a sequence of frequent hospitalization, now the patient is physically fit with CCM Therapy and GDMT.
The implantation method for a CCM device is fairly similar to that of a dual-chamber pacemaker, with the only variation being the placement of the two right ventricular leads, which are positioned in such a way (approximately 2cm apart septal) that influences LV function is assured.13 This above-mentioned Device is usually implanted on the right side sub clavicular Position because most of the time there is always one implanted ICD or CRT-D. A CCM device is typically made up of an implanted pulse generator (IPG) and a rechargeable battery. Signal delivery occurs through a variable number of leads, and this aspect has been the subject of extensive study and development since CCM was originally tested.
CCM is a novel HF device therapy, that works by delivering a high-energy, no excitatory biphasic signal during the absolute ventricular refractory phase of the cardiac cycle through two active fixation leads implanted on the right interventricular septum.14
The energy delivered by CCM is approximately a hundred times that of a standard pacemaker impulse, but these signals do not initiate depolarization because the stimulus is delivered during the absolute refractory period of ventricular cardiomyocytes, approximately 30-40ms after detection of local electrical activity (during phase 2 of the action potential of cardiac myocytes).15,16
In HF patients, the mechanism of action of CCM is mostly dependent on improved calcium management and normalization of pathologic HF gene expression. The phosphorylation of phospholamban improved calcium management by increasing calcium reuptake into the sarcoplasmic reticulum.17
Furthermore, patients who underwent CCM had their HF gene expression profile reversed (toward normal) (18). These two methods support the use of CCM treatment for patients with HFrEF since it improves cardiac contractility (in the absence of increased myocardial oxygen demand).19
CCM therapy was linked to improvements in exercise tolerance (both in the 6-min walk test and the peak VO2 in the spiroergometry), an increase in quality of life, a decrease in HF hospitalizations, and reverse remodeling of the left ventricle (reduction in left ventricular volumes and increase in LVEF).9,20
The European Society of Cardiology's current recommendations on the diagnosis and therapy of acute and chronic heart failure7 suggest that CCM should be considered in patients with HF, NYHA class III-IV HFs, an LVEF of 25% to 45%, and a QRS duration of 130 ms. The CCM approach is viable and might be used as a potentially valuable adjunct in CRT-NR when no other choices are available. In this critically ill group, mortality and clinical events remain high.(21) Considering our patient features (age of 72 years, ischemic dilated cardiomyopathy, pacemaker induced LBBB, NYHA class III-IV, and multiple HF-related hospitalizations), and CRT-Nonresponse, collectively the novel CCM device therapy was decided as a further plan of treatment. At 8months Follow up a very impressive enhancement of left ventricular function with reduced severity of multivalvular regurgitation. As a result, we believe that in older patients with HFrEF who have NYHA class III-IV, frequent HF-related hospitalizations, and a CRT-Nonresponse, CCM therapy can be considered to improve functional capacity, reduce hospitalizations, and reverse left ventricle remodeling so that left ventricular function improves.
We conclude that the CCM approach is practical and might be used as a potentially valuable adjunct in CRT Nonresponder (CRT-NR) with measured risks when no other choices are available. In this critically ill patient with frequent heart failure hospitalization, mortality, and clinical events remain high. After the CCM-Implantation, the betterment in Quality of Life (QoL), with no HF hospitalizations and considerable echocardiographic improvement in LVEF is proving the CCM Device Therapy as one of the best-emerging heart failure treatments. Long-term prognosis studies in a variety of heart failure groups are required to explain the specific role of CCM in modern cardiology and to determine whether the reported effects justified such a sophisticated and demanding treatment. These concerns are being addressed through multi-center research.
None.
Author Declare there are no Conflicts of interest.
None.

©2023 Mohapatra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.