Journal of
eISSN: 2373-4396


Research Article Volume 9 Issue 4
1Division of Cardiac Surgery, Hospital Universitari Germans Trias i Pujol, Spain
2Division of Cardiology, Hospital Universitari Germans Trias i Pujol, Badalona, Spain, School of Medicine, Universitat Aut
3Department of Preventive Medicine, School of Medicine, Universitat Aut
4Department of Surgery, School of Medicine, Universitat Aut
5Division of Cardiology and Thoracic and Vascular Sciences, Padova University, Italy
6Department of Surgery, School of Medicine, Universitat Aut
Correspondence: Bernardo Romero-Ferrer, Servicio de Cirugía Cardíaca, Hospital Universitari Germans Trias i Pujol, Carretera de Canyet, s/n, 08916 Badalona, Spain, Tel 34654894796
Received: July 08, 2017 | Published: July 27, 2017
Citation: Romero-Ferrer B, Lupón J, Casas-García I, Moret-Ruiz E, Colli A et al. (2017) Pulmonary Veins Epicardial Isolation with High-Intensity Focused Ultrasounds for the Treatment of Non-Primary Atrial Fibrillation. J Cardiol Curr Res 9(4): 00329. DOI: 10.15406/jccr.2017.09.00329
Background: Atrial fibrillation (AF) can be treated surgically by using high intensity- focused ultrasounds as an energy source with the Epicor system. The objectives of the present study are: first, to evaluate the effectiveness and safety of that technique at one year of follow-up; second, to establish the differences of the results depending on whether AF is paroxysmal or chronic and third, according to the underlying heart disease.
Methods: We treated 59 patients with the Epicor system, by doing a pulmonary veins epicardial ablation in patients with no primary AF operated on due to their cardiac disease. Patients were divided into two groups, one with 20 patients with paroxysmal AF and another with 39 chronic AF. Presence of sinus rhythm (SR) at first year was the primary endpoint and it was established according to EKG.
Results: The effectiveness to maintain SR was 67.8% in the operating room (OR), 54.4% at discharge, 54.7 at one month, 62% at six months and 65.3% at one year. No significant differences were found according to the underlying disease or the type of AF, although the results were better when AF was paroxysmal (at one year maintained SR up to 87.5% of mitral patients, 100% of aortic patients and 66.7% of ischemic patients). There were no technique-related serious complications.
Conclusion: Epicardial surgical ablation of the pulmonary veins with high-intensity focused ultrasounds is safe and shows good results, especially in patients with paroxysmal AF, and it should be offered when a surgical procedure is scheduled as a part of the surgical treatment.
Keywords: ablation, arrhythmia, atrial fibrillation, ultrasound, veins
AF, atrial fibrillation; SR, sinus rhythm; OR, operating room; HIFU, high-intensity focused ultrasounds; SD, standard deviation; LA, left atrium; Mm, millimeters; EF, ejection fraction; NSR, no sinus rhythm; SP, surgical procedure
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia.1 Several studies, including the Framingham one, show the association between AF and increased risk of mortality and morbidity.2‒8 It is also known that patients who present for cardiac surgery and who already have a history of AF, have a worse prognosis if it is not treated.9‒11 We know that valvular or coronary surgery is not sufficient per se to cure the arrhythmia. Thus, Raine reports that of 92 patients operated on for chronic mitral insufficiency and AF, only 8.5% were in sinus rhythm (SR) after the intervention.12 Haissaguerre took a fundamental step in understanding the pathogenesis of AF by demonstrating that it was generated by focal points as a trigger around pulmonary veins.13s From the surgical point of view, Cox and his team developed a first effective surgical treatment for AF14,15 with high efficiency but it was technically difficult, added extra time to surgery and carried out morbidity. In fact, the success rate reported by the same Cox is over 90%. Other authors, taking advantage of the development of new technologies and energy sources, adapted the original procedure making it simpler and less invasive.
Given the need to simplify the surgical procedure, and thanks to Haissaguerre’s work, the ablation of the pulmonary veins has raised as an alternative. The main advantage is that ablation can be done directly from the epicardium, without opening heart cavities or adding bypass or surgery time. There are different sources of energy to do the ablation, such as radiofrequency, cryoablation, microwave, laser or high intensity ultrasound (HIFU). HIFU release energy in a focal manner, avoiding the damage of surrounding tissues of the heart. The energy used is acoustic and managed in a targeted manner reaching 10 millimeters from the transducer surface. Beyond this point the energy is dissipated within the left atrial cavity without causing damage to near structures.16
The objectives of this study are four: First, to evaluate the effectiveness of exclusive epicardial ablation of pulmonary veins with HIFU in patients with non- primary AF in our environment. Second, to establish, if any, differences in the results between paroxysmal AF and chronic AF patients. Third, to establish, if any, differences in the results obtained according to the cardiac underlying disease of patients. And fourth, to assess the safety of the technique in terms of related complications and mortality.
This is a prospective observational study of a cohort of patients with non-primary AF operated on due to their underlying heart disease, in which an epicardial ablation of pulmonary veins with HIFU using the Epicor system has been added to surgical procedure.
Study population
Ours is a tertiary center which began its program of Cardiac Surgery in 2000. From March 2006 to March 2013, 71 patients with AF have been treated. We used different techniques and, specifically, 59 patients have been treated with HIFU using the Epicor system. What has been done is to complete the surgical treatment of the underlying disease with surgical ablation of arrhythmia.
Ethical considerations
This study was approved by the Ethics Committee Research Center, code Ultrasons01 / 2014, reference CEI PI-14-032 and has been prepared in accordance with international recommendations on clinical research (Declaration of Helsinki of the World Medical Association). All patients received verbal and written information about the surgery planned and the additional ablation procedure. All patients signed an informed consent.
Postoperative protocol
Until discharge, heart rhythm was monitored and recorded by means of an EKG. In the absence of formal contraindications, amiodarone was administered to patients for a month and anticoagulation with oral anticoagulants a minimum of 6months. External electrical cardioversion was not considered necessary during admission unless patients poorly tolerated arrhythmia. Once discharged, patients were followed-up at one month, six months and then every year. The heart rate was established according to surface EKG, therefore, there are not Holter monitoring or other systems data available. All visits were made in the Outpatient Clinic of Cardiac Surgery Division. The primary endpoint is the maintenance of SR to the first year of follow-up. In addition, data are available for maintenance of SR at 2 and 3years of intervention in certain patients.
Statistical analysis
Data were treated with SPSS version 19 software. Continuous variables followed a normal distribution and are described as mean and standard deviation. Categorical variables were described as proportions. During follow-up, heart rate was divided into two groups for analysis, the first consisting of patients with SR and the second by patients with no SR, whether or not AF. Differences between groups were stablished with the Student’s t test for continuous variables
And the test or Fisher's exact test, when indicated, for categorical variables. A result of less than 0.05 was considered statistically significant. The relationship between the variables and maintenance of SR at first year was stablished by univariate and multivariate logistic regression analysis. A Kaplan-Meier curve (AF drop in follow-up) for the overall group of patients was built. The log-rank was used as a comparison test.
Preoperative data
We treated a total of 59 patients, who were divided into two groups to assess differences in the results. The first group consists of 20 patients with a history of paroxysmal AF (33.9%). The second group consists of the 39 patients (66.1%) with chronic AF (persistent 5 [8.5%] and 34 [57.6%] permanent).17 The clinical characteristics of patients are shown in Table 1. Significant differences in the surgical risk and taking calcium channel blockers and antiplatelet drugs were found.
Perioperative data
The average duration of the ablation procedure was 574 (SD 21) seconds (between 537 and 650seconds), about 9 and a half minutes, with no differences between the two groups. The average size of UltraCinch was 10.78 (SD 1,451) (8 to 13) with a mode of 11 in 16 cases (27.1%). In patients with chronic AF, size was significantly higher (10.20 SD1.19 vs 11.08 SD1.49, p = 0.027).
Postoperative data
Complications and mortality
No patient died or suffered a complication related to the ablation procedure. Supplementary Table 1 describes the complications suffered by patients during admission. During follow-up, a pacemaker was implanted in two patients (3.63%) and five patients died (9.09%), one due to a peripheral gangrene of the lower limbs, one due to an acute myocardial infarction, one kidney failure, and one septic shock and in the fifth patient the cause of death was not identified. Three patients had a chronic AF and two paroxysmal AF (p = 0.900).
|
|
Paroxysmal AF (n=20) |
Chronic AF (n=39) |
p |
|
Age (years) |
66 (SD 9,09) |
65,28 (SD 12,10) |
0,8181 |
|
Gender (men) |
15 (75%) |
25 (64,10%) |
0,3962 |
|
Pathology |
|||
|
Mitral |
9 (45%) |
19 (48,70%) |
0,7872 |
|
Aortic |
5 (25%) |
12 (30,80%) |
0,6432 |
|
Coronary |
6 (30%) |
5 (12,80%) |
0,1592 |
|
Congenital |
0 |
3 (7,70%) |
0,5442 |
|
AF length (months) |
88,80 (SD 153,47) |
44,39 (SD 62,39) |
0,2531 |
|
LA size (mm) |
48,56 (SD 6,32) |
51,76 (SD 8,13) |
0,1521 |
|
EF% |
58,60 (SD 12,07) |
55,33 (SD 14,59) |
0,3931 |
|
Euroscore |
4,95 (SD 1,60) |
6,05 (SD 2,51) |
0,0461 |
|
Logistic Euroscore |
4,07 (SD 1,92) |
6,88 (SD 5,39) |
0,0051 |
|
Treatment |
|||
|
Digoxin |
7 (35%) |
17 (43,60%) |
0,5252 |
|
β-blockers |
5 (25%) |
12 (30,80%) |
0,6432 |
|
Calcium channel blockers |
2 (10%) |
15 (38%) |
0,0222 |
|
Antiplatelet |
8 (40%) |
3 (7,7%) |
0,0032 |
|
Oral anticoagulants |
15 (75%) |
33 (84,60%) |
0,4832 |
|
Amiodarone |
7 (35%) |
5 (12,80%) |
0,0512 |
|
Percutaneous ablation |
0 |
1 (2,60%) |
0,9002 |
|
Thromboembolism |
2 (10%) |
7 (17,90%) |
0,7042 |
Table 1 Differences between the group of patients with paroxysmal AF and the group of patients with chronic AF Results are expressed as mean and standard deviation or as number and percentage.
AF: Atrial Fibrillation; SD: Standard Deviation; LA: Left Atrium; EF: Ejection Fraction; 1: Student’s T test; 2: χ2 test
|
Complication |
n |
% |
|
Respiratory failure |
2 |
3,39% |
|
Renal failure |
6 |
10,17% |
|
Pericardial effusion |
1 |
1,69% |
|
Cardiac tamponade |
1 |
1,69% |
|
Low cardiac output |
1 |
1,69% |
|
Pleural effusion |
1 |
1,69% |
|
Bleeding and reoperation |
1 |
1,69% |
|
Mesenteric ischemia |
1 |
1,69% |
|
Adynamic ileus |
1 |
1,69% |
|
Wound infection |
1 |
1,69% |
|
Pacemaker |
2 |
3,39% |
Supplementary Table 1 Complications during admission
Heart rhythm during follow-up
Table 2 shows the results of the intervention at first year. Most patients (65.3%) are in SR, especially when AF is paroxysmal, although the difference did not reach statistical significance (p = 0.068). Moreover, more than half of patients with chronic AF are also in SR. Table 3 shows the results in the other moments of follow-up, from the operating room to 3years. At all times of follow-up, there are more patients in SR than in AF. Only at first month, the difference in keeping SR between patients with paroxysmal AF and chronic AF is statistically significant. Specifically at 3years we have data on 44 patients, being 56.8% of them in SR.
|
|
Sinus Rhythm |
Non Sinus Rhythm |
p |
|
Type of AF |
0,06 |
||
|
Paroxysmal |
14 (82,4%) |
3 (17,6%) |
|
|
Chronic |
18 (56,3%) |
14 (43,7%) |
|
|
Type of Pathology |
0,72 |
||
|
Mitral |
17 (70,8%) |
7 (29,2%) |
|
|
Aortic |
8 (66,7%) |
4 (33,3%) |
|
|
Coronary |
5 (50%) |
5 (50%) |
|
|
Congenital |
2 (66,7%) |
1 (33,3%) |
|
Table 2 Results at first year of intervention by type of AF and underlying pathology AF: Atrial Fibrillation
There wereno differences in the primary outcome at first year according to the underlying heart disease (Table 2) or other follow-up time (Table 3). Within each pathology there was no difference between patients with paroxysmal AF and chronic AF, not only at first year. It’s noteworthy that all aortic patients with paroxysmal AF remain in SR at first year, a result that is also kept the second year. But it is also noteworthy that 87.5% of mitral patients with paroxysmal AF are in SR (Supplementary Table 2). Especially discreet are the results of coronary patients, even with paroxysmal AF.
|
Operating Room |
Discharge |
1 Month |
6 Months |
2 Years |
3 Years |
|||||||||||||
|
SR |
NSR |
p |
SR |
NSR |
p |
SR |
NSR |
p |
SR |
NSR |
p |
SR |
NSR |
p |
SR |
NSR |
P |
|
|
Type of AF |
0,126 |
0,071 |
0,006 |
0,113 |
0,161 |
0.267 |
||||||||||||
|
Paroxysmal |
80% |
20% |
68,4% |
33,6% |
84,2% |
15,8% |
76,5% |
23,5% |
75% |
25% |
66,7% |
33,3% |
||||||
|
Chronic |
61,5% |
38,6% |
47,4% |
52,6% |
41,7% |
58,3% |
54,5% |
45,5% |
55,2% |
44,8% |
51,7% |
48,3% |
||||||
|
Type of Pathology |
0.085 |
0,887 |
0,669 |
0,726 |
0,673 |
0,737 |
||||||||||||
|
Mitral |
71% |
29% |
57% |
43% |
50% |
50% |
66% |
34% |
68% |
32% |
64% |
36% |
||||||
|
Aortic |
53% |
47% |
53% |
47% |
64% |
36% |
58% |
42% |
63% |
37% |
45% |
55% |
||||||
|
Coronary |
91% |
9% |
55% |
45% |
55% |
45% |
63% |
37% |
44% |
56% |
50% |
50% |
||||||
|
Congenital |
33,3% |
66,7% |
33,3% |
66,7% |
33.30% |
66,7% |
33,3% |
66,7% |
66,7% |
33,·% |
66,7% |
33,3% |
||||||
Table 3 Heart rhythm in operating room, at discharge, 1 month, 6 months, 2 years and 3 years
AF: Atrial Fibrillation; SR: Sinus Rhythm; NSR: Non Sinus Rhythm
|
Pathology |
Paroxysmal AF |
Chronic AF |
Overall |
p |
|
Mitral |
7 |
10 (62,5%) |
17 |
0,352 |
|
(87,5%) |
(70,8%) |
|||
|
Aortic |
3 |
5 |
8 (66,6%) |
0,491 |
|
(100%) |
(55,5%) |
|||
|
Coronary |
4 |
1 |
5 (50%) |
0,524 |
|
|
(66,6%) |
(25%) |
|
|
Supplementary Table 2 Patients in sinus rhythm at first year according to the underlying disease and type of AF
Univariate and multivariate analysis and Kaplan-Meier curve
In univariate logistic regression analysis, only Euroscore and size of the left atrium were significantly related to the maintenance of SR at first year, while age bordered statistical significance (Table 4). In multivariate analysis, the variables that were significant were the type of AF, age and gender (Table 5). Figure 1 shows the Kaplan-Meier curve (AF relapse during follow-up) for the overall group of patients.
|
|
OR |
95% CI |
p |
|
Paroxysmal AF |
3,63 |
0,87-15,15 |
0,068 |
|
Pathology |
0,721 |
||
|
Mitral |
1 |
||
|
Aortic |
0,82 |
0,18-3,64 |
0,798 |
|
Coronary |
0,41 |
0,09-1,88 |
0,253 |
|
Congenital |
0,82 |
0,06-10,61 |
0,882 |
|
Age |
0,93 |
0,87-1,00 |
0,052 |
|
Male gender |
3,41 |
0,65-17,8 |
0,146 |
|
Euroscore |
0,69 |
0,51-0,93 |
0,016 |
|
AF length (months) |
0,94 |
0,99-1,00 |
0,759 |
|
LA size (mm) |
0,86 |
0,75-0,97 |
0,017 |
|
EF |
1,04 |
0,99-1,09 |
0,085 |
|
CPB time (minutes) |
0,99 |
0,97-1,00 |
0,279 |
|
Cross clamp time (minutes) |
0,98 |
0,96-1,01 |
0,321 |
|
Length or surgical procedure (minutes) |
0,99 |
0.96-1,00 |
0,384 |
Table 4 Relationship of variables with the maintenance of sinus rhythm at first year. Univariate analysis
OR: Odds Ratio; CI: Confidence Interval; AF: Atrial Fibrillation; LA: Left Atrium; EF: Ejection Fraction; CPB: Cardiopulmary Bypass
|
|
OR |
95% CI |
p |
|
Paroxysmal AF |
5,46 |
1,12-26,51 |
0,035 |
|
Age |
0,91 |
0,85-0,99 |
0,027 |
|
Male gender |
7,36 |
1,10-49,22 |
0,039 |
|
LA size (mm) |
O,88 |
0,76-1,01 |
0,084 |
|
EF |
1,03 |
0,97-1,09 |
0,315 |
|
Euroscore |
0,91 |
0,48-1,70 |
0,760 |
Table 5 Relationship of variables with the maintenance of sinus rhythm at first year. Multivariate logistic regression analysis
AF: Atrial Fibrillation; LA: Left Atrium; EF: Ejection Fraction; OR: Odds Ratio; CI: Confidence Interval
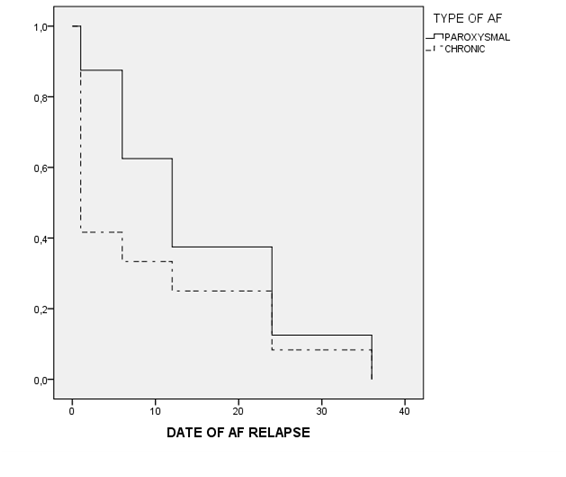
Figure 1 Kaplan-Meier curve (AF relapse during follow-up).
Log Rank 0,481 Among patients with SR after the ablation procedure, we can see that the recurrence of AF during follow-up is earlier in those patients with previous chronic AF compared to patients with previous paroxysmal AF, although the differences are not statistically significant.
AF is a common condition among patients who have to undergo cardiac surgery and it is associated with increased surgical morbidity and mortality.18‒21 In our study should be noted that the ablation procedure is added to the surgical procedure that each patient needs for his cardiac illness. Also, it does not increase the time of surgery, since ablation is performed while the surgeon prepares the patient for cardiopulmonary bypass. Overall, in all follow-up times there are more patients who maintain SR, specifically at first year 65.3% of patients are in SR. Our results are in line with those of the Spanish National Registry and at three years they are even better.22 And they are consistent with data from other studies. Thus, Schopka reports a maintenance SR at one year of 62%.23 Camm published a review of the most important articles in the literature with maintenance of SR after surgery and ablation procedure ranging from 60 and 82%.24 Even so, our results are lower than those shown in the best series published also using HIFU as an energy source.25,26 Groh reports maintenance of SR of 84.4% of patients at first year. Ninet’s study has a follow-up of six months, reaching maintenance of SR in 85% of patients and 100% when AF is paroxysmal.
We have also to keep in mind that our strategy has been to make only an ablation procedure around the pulmonary veins, regardless of the underlying disease and the need or not to open heart chambers. Therefore, it is the simplest ablation procedure that can currently be offered, without adding new lines of ablation, which could explain the better results of other studies. In this sense the FAST study is particularly interesting.27,28 Although radiofrequency energy source and not the HIFU was used in this study, no difference between surgical patients receiving mitral line mitral ablation compared to those who only received an ablation of the pulmonary veins was found, which would support our ablation strategy. Gillinov also studies the results of surgical radiofrequency ablation or cryoablation in mitral patients with paroxysmal AF. He concludes that the isolation of the pulmonary veins may be sufficient in these patients, especially when the AF is recent.29
In our series, the scenario is more favorable in the case of patients with paroxysmal AF. Thus, at first postoperative month and first year, 82.4% of patients with paroxysmal AF maintain SR. For patients with chronic AF, they maintain SR at first month up to 41.7%, at 6months 54.5% and at first year 56.3%. The literature also shows that the results are better in cases of paroxysmal AF. In Camm study up to 47% of patients with chronic AF maintain SR, but up to 85% of patients with paroxysmal AF remain in.15,24‒30 Pizón’s and Davies’ studies are the latest published using HIFU. Davies reports, at two years, 49% of patients maintaining SR, but according to the type of AF, patients with paroxysmal AF kept SR up to 81% of cases, patients with persistent AF up to 56% and patients with permanent AF up to 18% of cases.31 Pizón studied 78 patients. At 3 and 6 months remained in SR 66.7% and 100% of patients with paroxysmal AF, 100% and 90% of patients with persistent AF and 33.3% and 56% of patients with permanent AF. As predictors of the ablation outcome, Pizón found that patients who maintained sinus SR had a smaller left atrial diameter and a smaller left atrial area.32 In our study, the variables that were related to maintenance of SR at first year were Euroscore and the size of left atrium in the univariate analysis, while in multivariate analysis were paraxismal AF, age and male gender.
If we look at the results according to the underlying conditions of patients, in all cases but coronary patients at the end of surgery, SR is always more incident when AF is paroxysmal. In the case of coronary patients, our results are lower than those of the best series published, like Groh, reporting 85% of patients with coronary disease who maintain SR at one year of follow-up.33 Even so, more than half of coronary patients with paroxysmal AF RS remain in SR at first year in our study. We are especially interested in the results obtained in patients with mitral and aortic pathology. In fact, 100% of aortic patients with paroxysmal AF remain in SR until the second year and at third year up to 66.7% are in SR. And for mitral patients with paroxysmal AF, up to 90% are in SR at first year and 75% at second and third year.
In our series, no patient died or suffered any complication related to the ablation procedure. The most common complication was renal failure and no cases of stroke or thromboembolism were observed. In a recent meta-analysis, Phan concludes that adding an ablation procedure to mitral surgery significantly increases the rate of SR, without increasing mortality rates, need for permanent pacemaker, stroke or thromboembolism.34 Thus, the observed complications are those expected after a similar cardiac intervention but without the procedure ablation.35 The results of this study are in line with other studies which show that adding the AF ablation procedure to the planned surgery does not increase the surgical risk.2,36 Thereby, after the study of complications and mortality we can say that the technique is safe.
Although the difference is statistically significant only at first month, at one the year is quite significant. And although it is not significant neither at second nor at third year, this surgical ablation technique cannot be denied a priori to a patient presenting with a chronic AF, since at least half of treated patients will remain in SR.
Looking to the future
We can offer hybrid procedures to our patients, offering cardiologists and surgeons the opportunity of working together, combining in one procedure, a surgical epicardial ablation and a minimally invasive percutaneous endocardial ablation, using the best of both techniques. Surgeons would create ablation lines and cardiologists would evaluate and complete them when necessary, for example, when a line was not transmural. HIFU would be an excellent bet in this regard. This concept of hybrid procedure was proposed by Pak 37 and other authors have already described their results.38‒41
Limitations
The small number of patients included difficults to find significant differences. Also, this is an observational study, not a randomized experimental one because it does not compare to a control group. But the most important limitation is that the heart rate during follow-up has only been based on the surface EKG. Therefore, no data are available derived from Holter monitoring or implantable devices. It is possible that the incidence of patients in SR may be overestimated.
Epicardial surgical ablation of the pulmonary veins with HIFU is safe and shows good results at first year, especially in patients with paroxysmal AF and should be offered to patients with surgical indication for their underlying condition.
Authors would like to thank the Divisions of Cardiac Surgery and Cardiology at Hospital Universitari Germans Trias i Pujol for their support.
None.
Author declares there are no conflicts of interest.

©2017 Romero-Ferrer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.