Journal of
eISSN: 2373-4396


Case Series Volume 15 Issue 1
1Intensive Care Unit, Regional Clinical Hospital of Antofagasta,Chile
2Professor of Surgery, University of Antofagasta, Chile
3Department of Cardiovascular Surgery Antofagasta Regional Clinical Hospital, Chile
4Coronary Intensive Care Unit, Antofagasta Regional Clinical Hospital, Chile
5Respiratory Therapist of Coronary Intensve Care Unit, Antofagasta Regional Clinical Hospital, Chile
6Nurse of Coronary Intensive Care Unit, Hospital Clínico Regional de Antofagasta, Chile
7Surgery Fellowship, University of Antofagasta, Chile
Correspondence: Vinko Tomicic, Unidad de Cuidados Intensivos 2° piso Hospital Clínico Regional de Antofagasta, Azapa 5935, Antofagasta, Chile, Tel +56227583969
Received: December 09, 2021 | Published: January 25, 2022
Citation: Tomicic V, Labbé J, Pumarino A, et al. Pilot experience with VV ECLS in a single hospital in a developing nation. J Cardiol Curr Res. 2022;15(1):4-7. DOI: 10.15406/jccr.2022.15.00541
Pilot experience with VV ECLS in a single hospital in a developing Introduction: Worldwide there are more than 160million confirmed cases of SARS CoV-2 infection and more than 3million deaths this year, of which 47% correspond to the Americas.1 About 4% of patients require mechanical ventilation (MV) and mortality can reach more than 40%.2 Some patients may present with refractory respiratory failure (RRF) that requires extracorporeal life support (ECLS). In Chile, in the second outbreak of SARS CoV-2, the incidence of the young population that evolved with RRF increased, which prompted us to coordinate the transfer of patients from their centers of origin (northern macrozone) and implement the VV ECLS in the Coronary Intensive Care Unit (UCO) of the hospital clinico regional de Antofagasta (HCR). Methods: Two ECMO teams were organized, each with 1 heart surgeon, 1 cardiovascular anesthetist, 1 perfusionist, and 1 surgeon’s assistant. The patients were transferred assisted with the Cardiohelp system, Maquet (oxygenator [Quadrox-iR]). Ground transfers were carried out in SAMU ambulances and air transfers in planes arranged by health ministry. Resuts: Ten patients were admitted (6 from Calama, 1 from Iquique, 1 from Arica, 1 from Santiago de Chile and 1 of our hospital), 8 male. The median age was 35.5. The APACHE II and SOFA on admission were 18.5 and 12, respectively. The VM pre-ECLS stay was 8 days.1–10 The ventilatory setting at admission to VV ECLS was VT 3 [2.2-5] ml/kg PBW, respiratory rate (RR) between 10-20, driving pressure 11.5 [8-19]; inspiratory time: 2 [0.9-2.5] seconds; plateau pressure: 27; FiO2 ≤50 and PEEP 16 cmH2O and PEEP 16cmH2O. Three patients had serious bleeding complications that contraindicated systemic anticoagulation: 1. upper gastrointestinal bleeding due to gastric stress ulcers; 2. spontaneous acute subdural hematoma on the third day of VV ECLS, and 3. profuse oronasal bleeding with a drop in hematocrit, despite anterior and posterior tamponade, and seeking TTPK between 1 to 1.5 times the baseline value, here we opted for extracorporeal CO2 removal without a pump (P-ELA: pumpless-extracorporeal life assist) with regional anticoagulation. Two patients evolved with static compliance of 10ml/cmH2O, forcing to use ultra-protective MV and P-ELA had to be prolonged. In them, a pulse of 125mg/day methylprednisolone was decided with a good response. Static compliance, in both cases improved approximately in 7days.
Conclusion: The VV ECLS in patients with RRF due to SARS-CoV-2 pneumonia is feasible to implement in a tertiary regional hospital. Taking on this challenge it was possible to offer ECLS to patients from all over the north of Chile, who previously had to wait for a place in other centers far from the north. In patients with active bleeding, bridge to P-ELA offers the possibility of regional anticoagulation.
Keywords: SARS-CoV-2, refractory respiratory failure, mechanical ventilation, extracorporeal blood flow, hemoglobin, SvO2
MV, mechanical ventilation; RRF, refractory respiratory failure; ECLS, extracorporeal life support; RR, respiratory rate; BAL, bronchoalveolar lavage
Worldwide there are more than 160million confirmed cases of SARS CoV-2 infection and more than 3 million deaths this year, of which 47% correspond to the Americas.1 About 4% of patients require mechanical ventilation (MV) and mortality can reach more than 40%.2 Some patients may present with refractory respiratory failure (RRF) that requires extracorporeal life support (ECLS).3–6
In Chile, in the second outbreak of SARS CoV-2, the incidence of the young population that evolved with RRF increased, which prompted us to coordinate the transfer of patients from their centers of origin (northern macrozone) and implement the VV ECLS in the Coronary Intensive Care Unit (UCO) of the hospital clinico regional de Antofagasta (HCR).
In Chile, ECLS support only was given in the central and south of the country; so, to begin this program in the north is been a great progress, especially during the pandemia. From Santiago to Arica, the northest city of our country, there are 2,400kilometers without this support strategy for the young patients with RRF. We describe then, the evolution and results of our first 10 patients assisted with VV ECLS.
We describe the patients who were admitted at the HRA in UCO with a diagnosis of RRF that required a transfer in VV ECLS from their centers of origin, who were admitted between March and September 2021. These patients were selected by the commission of ECMO National Minsal and evaluated by the HRA ECMO team.
The VV ECLS is capable of temporarily replacing respiratory function when management with advanced ventilatory support strategies fails (PaO2/FiO2 <100mmHg with FiO2≥60% or hypercapnia with pH≤7.2).5
Two ECMO teams were organized, each with 1 heart surgeon, 1 cardiovascular anesthetist, 1 perfusionist, and 1 surgeon’s assistant. With the experience of UCO in post-operative venoarterial ECLS (VA-ECLS), there were nurses prepared to 2 shifts. The patients were transferred assisted with the Cardiohelp system, Maquet Getinge (integrated module with oxygenator [Quadrox-iR] and centrifugal pump). Ground transfers were carried out in SAMU ambulances and air transfers in planes arranged by health ministry (MINSAL).
Age, APACHE II, SOFA, pre-ECLS MV days, ECLS days, MV days, ICU days, hospital days and outcomes were collected. At admission, daily and every 6hours the respiratory mechanics (physiotherapist), arterial blood gases and the data provided by the Cardiohelp system console (extracorporeal blood flow, hemoglobin, SvO2) and FiO2 from the blender and sweep flow were collected by the nurse and the perfusionist checking also the circuit. The main complications were recorded.
The study was approved by the ethics committee of the Antofagasta Health Service. All patients or next of kin gave their consent to communicate the data respecting the identity of the patients.
Ten patients were admitted (6 from Calama, 1 from Iquique, 1 from Arica, 1 from Santiago de Chile and 1 of our hospital), 8 male. The median age was 35.5 [31-63]. The APACHE II and SOFA on admission were 18.5 [17-23] and 12 [7-17], respectively. The VM pre-ECLS stay was 8 [1-10] days. Upon admission to UCO, while in ECLS (100% blender and sweep flow 5L/min), the median PaO2/FiO2 and PaCO2 are described in Table 1.
|
Patient |
Age |
ACHEII |
Sofa |
aO2/FiO2 |
actate |
FiO2/ |
PaCO2 |
VMI (d)PRE-ECLS |
ECLS (d) |
P-ELA |
MV(d) |
ICU (d) |
Hosp (d) |
Outcome |
|
t |
46 |
19 |
14 |
74 |
8,6 |
1 |
52 |
10 |
8 |
No |
38 |
50 |
50 |
Alive |
|
2 |
32 |
23 |
16 |
87 |
15,2 |
0,95 |
38 |
1 |
12 |
SI |
60 |
6d |
64 |
Alive |
|
3 |
47 |
17 |
12 |
86 |
7,8 |
0,9 |
39,5 |
9 |
12 |
No |
67 |
79 |
79 |
Alive |
|
d |
34 |
17 |
14 |
74 |
16 |
0,9 |
41,4 |
3 |
17 |
SI |
38 |
45 |
45 |
Alive |
|
5 |
32 |
17 |
12 |
90 |
12,4 |
0,95 |
28,3 |
9 |
5 |
SI |
33 |
52 |
52
|
Alive |
|
b |
32 |
19 |
17 |
71 |
17,1 |
1 |
51,1 |
7 |
4 |
NO |
32 |
39 |
39 |
Dead |
|
7 |
37 |
18 |
12 |
58 |
l4 |
1 |
53 |
6 |
7 |
No |
13 |
l9 |
19 |
Dead |
|
g |
31 |
21 |
12 |
73 |
18,2 |
I |
44,9 |
8 |
10 |
No |
31 |
49 |
49 |
Alive |
|
g |
46 |
16 |
7 |
67 |
9,7 |
1 |
55 |
8 |
37 |
No |
45 |
53 |
53 |
Dead |
|
Jp |
63 |
20 |
8 |
49 |
15.5 |
1 |
67,3 |
2 |
26 |
SI |
63 |
63 |
|
Dead |
|
Median(R) |
35,5 (31-63) |
18,5 (17-23) |
12 (7-17) |
73,5 (49-90 |
14(7,8-18,2) |
1 (0,9-1) |
48(28,3 -67,3) |
7,5 (1-10) |
11 (4-37) |
4/10 |
38 (13-67) |
51 (19-79) |
50 (19-79) |
7/10 |
Table 1 Admission parameters and evolution of patients with VV ECLS in coronary ICU
ECLS: extracorporeal life support; APACHE II: Acute Physiology and Cronic Health Evaluation II, SOFA: Sequencial Organ Failure Assessment; FiO2: Inspired oxygen fraction; VM: Mechanical Ventilation; P-ELA: pumpless-extracorporeal life assist; ICU: Intensive Care Unit; (d): days; LACTATE: mg/dL; PaCO2: mmHg
Weaning: in espontaneous ventilation by tracheostomy. Admission variables are axpressed in median range
The ventilatory setting at admission to VV ECLS was VT 3 [2.2-5] ml/kg PBW, respiratory rate (RR) between 10-20, driving pressure 11.5 [8-19]; inspiratory time: 2 [0.9-2.5] seconds; plateau pressure: 27 [19-37]; FiO2 ≤50% and PEEP 16 [8-21] cmH2O [6]. The ICU and hospital lengh of stay are the same because the patients were discharged the hospital from the ICU.
In the original center, our team supinated the patients prior to cannulation, which was performed by the right internal jugular vein (arterial cannula HLS OD=19 Fr [6.3mm] 15cm) and right femoral vein with venous cannula HLS OD=21-29 Fr [7.0mm] 55cm. Raumedic® 3/8-3/32 lines were used. The evolution of arterial blood gases and extracorporeal support during the first two weeks are expressed in median range and is described in Figure 1.
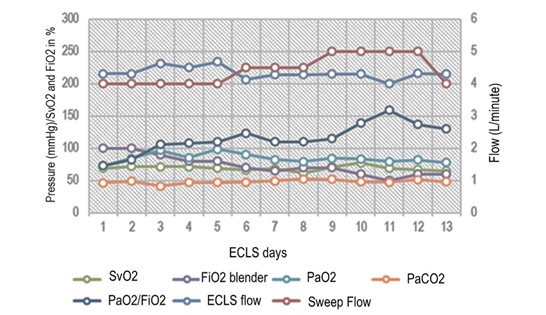
Figure 1 ECLS and blood gas parameters.
Left axis describe blood gas parameters (mmH g) and venous saturation from de Cardiohel p console (%). Right axis show ECLS Flow and Sweep FI ow in Litres per minute (L/min).
SvO2, pre-oxygenator venous oxygen saturation; PaCO2, arterial pressure of CO2; PaO2/FiO2, pressure of arterial oxygen to fractional inspired oxygen concentration; fraction of oxygen from the extracorporeal circuit [blender].
Upon in our ICU, two patients despite receiving 5 liters of extracorporeal blood flow, the blender with 100% oxygen and with 100% oxygen in the ventilator, persisted with saturation ≤90%, for which they underwent through pulmonary recruitment maneuvers (LRM) of rescue with pressures up to 45cmH2O as described.7,8 After recruitment, saturation rose above 90%.
Three patients due to stationary evolution of oxigenation required to be pronated with the extracorporeal circuit, with which an additional improvement in oxygenation was achieved and they returned to supine in approximately 48 hours.9
Three other patients had serious bleeding complications that contraindicated systemic anticoagulation: 1. upper gastrointestinal bleeding due to gastric stress ulcers; 2. spontaneous acute subdural hematoma on the third day of VV ECLS, and 3. profuse oronasal bleeding with a drop in hematocrit, despite anterior and posterior tamponade, and seeking TTPK between 1 to 1.5 times the baseline value.
In these three patients, the ability of the native lung to contribute to oxygenation was previously estimated and, if possible to ventilate without transgressing the driving pressure, we opted for extracorporeal CO2 removal without a pump (P-ELA: pumpless-extracorporeal life assist) with regional anticoagulation installing the heparin infusion pump in the arterial line (before the CO2 extractor) and the protamine pump in the venous line before entering the patient's circulation. The heparin/protamine ratio was 2:1, with which the systemic TTPK remained normal and stable. The upper gastrointestinal bleeding was resolved endoscopically without problems, the acute subdural hematoma (SDH) was drained while P-ELA was working, without complications. Tomographic monitoring of the brain was carried out dayly and no needed a new intervention. Oronasal bleeding stopped once the system with regional anticoagulation was established.
One patient developed cholangitis with acute biliary edematous pancreatitis, he was treated with imipenem and post-ECLS, endoscopic retrograde cholangio-pancreatography was performed, removing stones with good evolution. One of the patients had positive bronchoalveolar lavage (BAL) for tuberculosis and IV therapy was started.
Two patients evolved with static compliance of 10ml/cmH2O, forcing to use ultra-protective MV and P-ELA had to be prolonged.10,11 In them, a pulse of 125mg/day methylprednisolone was decided for three consecutive days with a good response. Static compliance, in both cases increased around 10ml/cmH2O, approximately in 7 days.12
Six patients showed thrombocytopenia after the 3rd day of ECLS and in 3, the platelet count reached values lower than 50,000mm3, therefore, the heparin infusion was stopped and the extracorporeal blood flow was maintained above 5 l/min. Partial coagulation of the oxygenator was observed in one patient, which was changed without incident.
Seven patients survived and were discharged. The patient with SDH presented mild left paresis without cognitive impairment. Three died, one of them, in his brain computed tomography on admission, showed cerebral edema and finally brain death was confirmed and the ECLS was removed. Two, being out of ECLS, presented sepsis due to gram-negative bacilli and died by refractory septic shock.
The VV ECLS is a safe strategy that allows to apply rest ventilation, while the lung recovers from the insult that motivated the RRF, as it can be deduced from national and international studies.4–6,10–13 With P-ELA and regional anticoagulation, it was possible to stabilize bleeding in the cases described and maintain resting ventilation at the same time. During this process, saturations between 89 and 91% were tolerated and it was tried to reach a ScvO2 over 65%. The latter was achieved with dobutamine at 2-3µg/kg/min and in case of arterial hypertension with milrinone at 0.3µg/kg/min, a crucial indication to maintain oxygen transport when leaving VV ECLS early. It was possible to achieve blood flow in the P-ELA circuit between 1.20 and 1.78 L/min and the sweep flow were maintained between 5 and 8L/min.10 Ventilatory setting was maintained with VT between 2.5 and 3.5ml/kg of PBW, RR between 10 and 20 and a PEEP between 8-16 cmH2O.6,10,11
Although according to the literature, thrombocytopenia would not be associated with the duration of ECMO, in our patients it was more noticeable after the first week. A meta-analysis found that the prevalence of thrombocytopenia in VV ECLS was 25.4% (95% CI: 10.6-61.4) in data obtained from 4 studies. The prevalence of heparin-induced thrombocytopenia (HIT) was 3.7% (95% CI: 1.8-5.5) and platelet dysfunction was described in seven studies.14 Thrombocytopenia and platelet dysfunction are common in patients undergoing ECLS, and the mechanisms are multifactorial.
The VV ECLS in patients with RRF due to SARS-CoV-2 pneumonia is feasible to implement in a tertiary regional hospital. Taking on this challenge it was possible to offer ECLS to patients from all over the north of Chile, who previously had to wait for a place in other centers far from the north macrozone. In patients with active bleeding, bridge to P-ELA offers the possibility of regional anticoagulation.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Thanks to all the nursing and paramedical staff who participated in the care of our patients who required ECMO.
The authors declare that they have no conflicts of interest.
None.

©2022 Tomicic, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.