Journal of
eISSN: 2373-4396


Case Report Volume 15 Issue 2
1Department of Cardiology, Hamad Medical Corporation, Qatar
2Department of Internal Medicine, Hamad Medical Corporation, Qatar
Correspondence: Ashraf Omer Elamin Ahmed, Department of Internal Medicine, Hamad Medical Corporation Doha, Qatar, Tel 0097455455894
Received: March 06, 2022 | Published: March 17, 2022
Citation: Abdullatef WK, Ahmed AOE, Shawky AH, et al. Left ventricular non-compaction masquerading as recurrent transient ischemic attacks: a case report. J Cardiol Curr Res. 2022;15(2):32-34. DOI: 10.15406/jccr.2022.15.00548
Isolated left ventricular noncompaction (LVNC) is an uncommon cause of structural heart disease. Its main features are deep intertrabecular recesses and abnormal trabeculations of the left ventricle. These structural changes are seen by the transthoracic echocardiogram (TTE) or the cardiac MRI (CMR). The clinical manifestations are systolic or diastolic dysfunction, conduction abnormalities, and cardioembolic events theorized to result from thrombus formation within the intertrabecular recesses. The management of LVNC is individualized based on the presenting symptoms. In this report, we are presenting a young female who presented with recurrent episodes of transient ischemic attack (TIA), conduction abnormalities, and moderately reduced left ventricular ejection fraction (LVEF), along with a literature review.
Keywords: left ventricular non-compaction, embolic stroke, cerebrovascular accidents
LVNC, left ventricular noncompaction; CT, computed tomography; TIAs, transient ischemic attacks; HCM, hypertrophic cardiomyopathy; CMR, cardiac magnetic resonance; TTE, transthoracic echocardiography with contrast
Left ventricular noncompaction (LVNC) is an uncommon etiology of cardiomyopathy. The prevalence of LVNC is estimated to be 0.014 and 1.3 percent in the general population. The hyper-trabeculations are assumed to be due to a developmental error during which the typical myocardium compacts inward or while the ventricular wall is formed.1,2 The clinical manifestations vary widely and range from asymptomatic to symptoms of diastolic dysfunction, systemic cardioembolism, and conduction abnormalities.2 Currently, there are no universal criteria for classifying LVNC. Depending on the organization, it may be classified as genetic cardiomyopathy or as an unclassified cardiomyopathy.3,4
The diagnosis of LVNC is established by viewing the structural abnormalities using cardiac imaging studies such as echocardiography, computed tomography, or CMR. The Jenni, Chin, and Stollberger criteria emphasize different echocardiologic manifestations that might aid in the diagnosis.5 The diagnosis will rely on the presence of evidence of a two-layered structure (thin compacted epicardial layer and thick endocardial layer) with a non-compacted to the compacted ratio of greater than 2 (greater than 2.3 on CMR), an epicardial-to-trabeculation trough to epicardial-to-trabeculation peak ratio of less than 0.5, and more than three trabeculations from the ventricular wall (not sensitive for CMR).6,7
A 38-year-old lady known to have non-obstructive hypertrophic cardiomyopathy (HCM) was referred to the cardiology clinic after three transient ischemic attacks (TIAs). The first episode manifested as right-side weakness and numbness for 45minutes which resolved ultimately. Since then, she was started on Aspirin and statin. The other two episodes of TIA were ten and twelve months later, as she experienced a similar episode of around 30-minute duration while she was on Aspirin, which also resolved completely. She underwent a CT head which was normal. MRI head was done and showed subacute infarcts in the left basal ganglia, insula, and left frontal cortex. An embolic nature of the stroke was suspected as it was distal and cortical; thus, she was referred to our clinic.
She provided a history of atypical chest pain, exertional shortness of breath, and occasional palpitation on further assessment—no lower limbs edema or syncope. Physical examination was utterly unremarkable. ECG (Figure 1) revealed brad ectopic pacemaker rhythm in lower atrial focus, Q waves in inferior leads with R wave in V1-V2, and nonspecific ST-T changes. Transthoracic echocardiography with contrast (TTE) revealed a thickened LV with more than three trabeculations protruding from the posterolateral wall. These moved synchronously with the myocardium (Figure 2). Intertrabecular recesses were evident, and the systolic function was moderately reduced (LV ejection fraction, 0.37), with a thick basal-mid septum of 1.9cm. The rest of the LV wall is hyper-trabeculated (Figure 2). Cardiac MRI showed thick basal-mid septum (1.9cm), hyper trabeculated LV with Peterson et al. criteria of 2.5 in keeping morphically with the diagnosis of LVNC. A patchy diffused mid-wall myocardial enhancement from base to apex was noticed indicates myocardial fibrosis. No cardiac thrombi were appreciated (Figure 3).
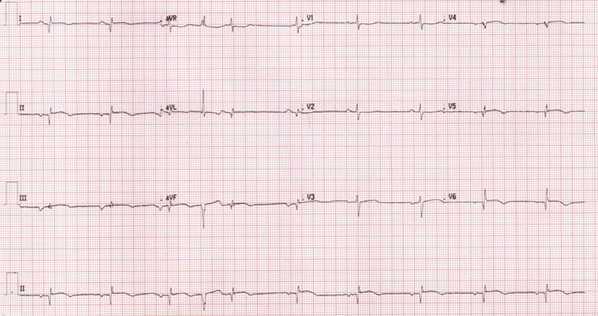
Figure 1 Revealed brad ectopic pacemaker rhythm in lower atrial focus, Q waves in inferior leads with R wave in V1-V2, and nonspecific ST-T changes.
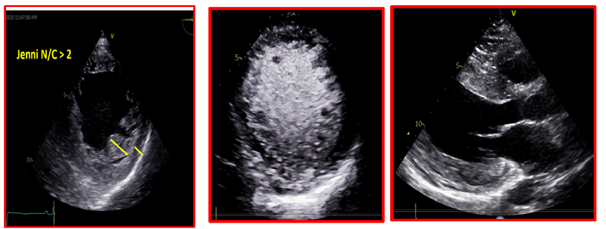
Figure 2 TTE revealed a thickened LV with more than three trabeculations protruding from the posterolateral wall. These moved synchronously with the myocardium. Intertrabecular recesses were evident.
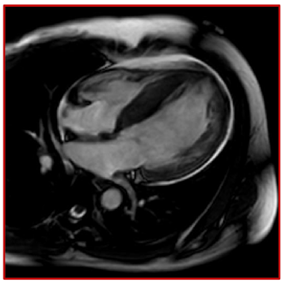
Figure 3 Suboptimal CMR, however; it showed thick basal-mid septum (1.9 cm), hyper-trabeculated LV, and patchy diffuse enhancement from base to apex indicative of myocardial fibrosis.
24-hour Holter monitor records NSVT, high burden PVCs (17%), multiple PVCs morphology, and non-sustained run of atrial ectopy. Jenni’s criteria diagnosed LVNC; the ratio of non-compacted NC to compacted C layer in systole is 2.1 (Yanni et al.) and MRI Peterson criteria.8,9
The electrophysiology team reviewed the patient, and dual champers intracardiac device ICD was inserted later on for primary prevention for HCM with more than 5% risk over five years using European Society of Cardiology risk score.10
She was started on Rivaroxaban 15mg and then switched to warfarin based on the literature and heart failure medications. The patient has been followed up for one year with on recurrence of the TIAs. The ICD interrogated on the follow-ups and showed 17 events recorded under VT/ NSVT zone; however, no shocks were delivered.
Cardiomyopathy has different etiologies, with LVNC being one of the uncommon causes. It is characterized by prominent trabeculations and intertrabecular recesses that communicate within the ventricular cavity.11,12 The condition affects the LV; however, it might also be biventricular. It is assumed to be caused by the arrested development of the myocardial compaction between weeks five and eight of the embryogenesis.13 The most frequently affected myocardial segments are the apex, the distal, and the middle segments of the inferior and lateral walls. The affected segments have a 2-layered structure: a thin compacted pericardial layer and a thicker noncompacted endocardial layer with deep recesses— the basis of the LV noncompaction definition (Figure 2).14
The diagnosis of LVNC is challenging; in one series, the diagnosis of LVNC was initially missed in 89% of the cases: 42% of the cases were dilated cardiomyopathy, and 15% were hypertrophic cardiomyopathy.15 Isolated LV noncompaction is diagnosed by cardiac imaging, the TTE, and cardiac magnetic resonance (CMR). However, contrast echocardiography, 3-dimensional echocardiography, transesophageal echocardiography, multidetector computed tomography, and LV angiography might reveal the deep intertrabecular recesses and help in the diagnosis.16–18 Prominent trabeculations of the left ventricle can be found in up to 68% of the general population, which is considered a normal variant.18 The anatomic distribution of the trabeculations can be a helpful criterion for the diagnosis in that group because the trabeculations course from the free wall to the ventricular septum in 85% of LVNC cases (Figure 3).18
Since LVNC was firstly described, it has been associated with cardioembolic complications, and the embolic phenomenon was proposed to be due to thrombus formation within the intertrabecular recesses.19–21 However, Other articles have reported that concomitant cardiovascular or coagulation disorders that increase the risk for systemic embolisms—such as patent foramen oval, atrial fibrillation, atrial flutter, LV systolic dysfunction, antiphospholipid syndrome, elevated factor VIII levels, sepsis, or essential thrombocythemia.22–24
The management question will focus on the presenting scenario, whether arrhythmia, heart failure, or systemic embolism. Arrhythmias are usually treated with β-blockers, calcium-channel blockers, or antiarrhythmic drugs. Additionally, ICD and CRT are also considered to prevent sudden cardiac death.25 There is no clear Conesus regarding the anticoagulative therapy, yet, it has been proposed to start anticoagulation once there is evidence of the cardioembolic phenomenon. Warfarin was the anticoagulation proposed in the literature; however, aspirin was used for the same purpose.25
As suggested in our patient’s case, the development of a recurrent thromboembolic phenomenon and systolic dysfunction warrants prophylactic anticoagulation and heart failure management. The high burden of multifocal premature ventricular contractions raised a concern of increased risk for a sudden cardiac and fetal arrhythmia that warrants an intracardiac device for primary prevention.25
In summary, LVNC is an uncommon cause of cardiomyopathy. Although developmental and genetic factors have been proposed to be their underlying cause, the pathophysiology is unclear. It has variable clinical manifestations; thus, the management should be individualized based on the presenting symptoms, arrhythmias, heart failure, or cardioembolic phenomenon.
None.
None declared by the authors relevant to this article.
No funding sought for the writing or publication of this paper.
Patient provided consent to share his case.

©2022 Abdullatef, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.