Journal of
eISSN: 2373-4396


Research Article Volume 15 Issue 2
1Department of Cardiology, Cardiologicum Hamburg, Hamburg, Germany
2Department of Cardiology, University Heart Center Lübeck, Germany
3Department of E. Meshalkin National Medical Research Center of the Ministry of Health of the Russian Federation, Russia
Correspondence: Martin W. Bergmann, Cardiologicum Hamburg, Schloßgarten 3-7, 22401 Hamburg, Germany , Tel +494068280674, Fax +494068280675
Received: February 12, 2022 | Published: March 28, 2022
Citation: Paitazoglou C, Bergmann MW, Losik D, et al. Intramyocardial injections of erythropoietin-analogue C.E.R.A. in ischemic cardiomyopathy: the ALSTER C.E.R.A. trial. J Cardiol Curr Res. 2022;15(2):36-43. DOI: 10.15406/jccr.2022.15.00549
Objectives: Erythropoietin (EPO) improved cardiac regeneration in experimental models of ischemic heart disease. A pilot trial found subcutaneously administered EPO to improve surrogate markers of left ventricular (LV) function in ischemic cardiomyopathy. This clinical study tests the feasibility and safety of the intramyocardial delivery route of a long-acting EPO-analogue (C.E.R.A.) in patients with ischemic cardiomyopathy.
Methods: The ALSTER C.E.R.A. trial was a Phase II, open label, 1:1 randomized, single-center study testing intramyocardial injections of long-acting EPO analogue C.E.R.A. (C.E.R.A. NOGA: once 180 µg) using the NOGA XP system versus the subcutaneous application (C.E.R.A. SC: 30µg s.c./month for 6 months) in 59 symptomatic chronic heart failure (HF) patients with impaired LV function (ejection fraction (EF) £ 45%).
Results: Follow-up up to three years with both clinical and imaging endpoints found intramyocardial delivery of C.E.R.A. to be feasible, safe and to possibly attenuate LV remodeling. Patients in the C.E.R.A. NOGA group showed stable parameter for LV end-diastolic diameter and volume (LVEDD and LVEDV), while C.E.R.A. SC patients had significant dilation of the LV (C.E.R.A. NOGA vs. SC, mean ± standard error of the mean: DLVEDD 0.02±0.1mm, p=0.8 vs. 0.3±0.09mm, p=0.0026; DLVEDV 10±15.9ml, p=0.5 vs. 34.8±11.3ml, p=0.0081; ∆EF 2.4±1.2%, p=0.045 vs. -1.6±1.1, p=0.1 respectively). NYHA class significantly improved and the hospitalization rate was numerically reduced in the C.E.R.A. NOGA group, while three-year mortality was identical.
Conclusions: Intramyocardial injection of C.E.R.A. is feasible, safe and possibly attenuates LV remodeling in ischemic HF patients with LV dysfunction compared to the systemic application.
Keywords: heart failure, ischemic cardiomyopathy, erythropoietin, cardiac regeneration
C.E.R.A, continuous erythropoietin receptor activator; EF, ejection fraction; EPO, erythropoietin; HF, heart failure; LV, left ventricle; LV-EDD, left ventricle end-diastolic diameter; LV-EDV, left ventricle end-diastolic volume; MACE, major adverse cardiovascular events; WMSI, wall motion score index; 6MWD, 6-minute walking distance
Erythropoietin (EPO) is the main hematopoietic hormone acting on progenitor red blood cells via stimulation of cell growth, differentiation and anti-apoptosis. In addition, the EPO receptor is also expressed in various non-hematopoietic tissues, including endothelium and cardiac progenitor cells.1,2 EPO is a pleiotropic growth factor that exhibits growth stimulation and protection against ischemia on numerous cells and tissues.2 In a preclinical study, EPO induced neovascularization3 and an EPO-responsive cardiac progenitor cells population in the adult heart has been previously described.1 Low-dose EPO treatment with its non-hematopoietic effects not aiming to raise the hemoglobin level, but boosting endogenous repair mechanisms in response to hypoxia4 has led to many trials, investigating the administration of EPO early after myocardial infarction to prevent adverse cardiac remodeling and reduce clinical events after successful percutaneous coronary intervention, but results have been controversial.5–7
However, we found a repetitive low-dose epoetin-b subcutaneously treatment in patients with chronic ischemic heart disease (ejection fraction (EF) ³45%) to have beneficial effects on LV remodeling in a small pilot trial.8 To evaluate the feasibility and safety of a targeted injection of a long-lasting EPO-analogue in the peri-ischemic region in chronic symptomatic HF patients with LV dysfunction (EF ≤45%), we now randomized patients in a 1:1 pattern to receive either NOGA-guided intramyocardial injections of C.E.R.A. (180µg once) or subcutaneous application of C.E.R.A. for 6 months (180 µg in total) with a follow-up up to three years.
Study population
The ALSTER C.E.R.A. Trial was a Phase II, prospective, randomized open label study. The study complies with the Declaration of Helsinki and the locally appointed ethics committee approved the research protocol. Patients were eligible for the trial if they had an ischemic cardiomyopathy with a previous (>12months) successful percutaneous coronary intervention or a coronary artery bypass grafting and presented with symptomatic HF NYHA class ≥II and LV EF ≤45%. All patients included were on maximal tolerable standard HF medication and without further treatment options like valvular intervention/surgery or revascularization or implantation of a cardiac resynchronization therapy device. Investigators were encouraged to maintain patients on the maximal tolerable HF treatment regime during the follow-up period. Cardiac resynchronization therapy device implantation between baseline and the three-years follow-up would lead to study exclusion due to a possible effect on the clinical and imaging endpoints. Patients were asked for informed consent if they fulfilled the inclusion criteria and did not exhibit any of the following exclusion criteria: valvular heart disease (significant >grade 2: aortic stenosis/insufficiency or mitral- or tricuspid valve insufficiency), uncontrolled hypertension (resting blood pressure > 180/100 mmHg), hemoglobin > 16mg/dL. Patients aged >75years and pre-menopausal women were not included. Patients with active malignancy, severe renal dysfunction (serum creatinine >3.0mg/dL), chronic liver disease, or indication for open-label EPO treatment were also excluded. Patients with iron deficiency (ferritin < 50mg/mL and/or transferrin saturation < 20%) or anemia (hemoglobin < 12mg/dL) and patients with neurological diseases, including a prior stroke, were also excluded. Serum vitamin B12 levels were required to be within the normal range. The presence of a LV thrombus or an acute coronary syndrome within 12months prior to inclusion were also reasons for study exclusion.
Study protocol
Patients were screened and treated at the National Medical Research Center of the Ministry of Health of the Russian Federation, Novosibirsk. Eligible patients with signed informed consent, approved for the study were randomized in a 1:1 pattern to receive intramyocardial injections of C.E.R.A. (Continuous Erythropoietin Receptor Activator, 180µg in total) or subcutaneous application of C.E.R.A. (30µg /month) for six months. Follow-up visits were performed at six months and three years after study inclusion for safety and outcome evaluation with imaging and clinical endpoints. Imaging endpoints were the change (from baseline to three years) in echocardiography determined LVEDV, LVEDD and LV-EF (biplane). Furthermore, we assessed the diastolic parameter E/A and the echocardiographic wall motion score index (WMSI). Clinical endpoints were changes in functional NYHA class, 6-minute walking distance (6MWD) and major adverse cardiovascular events (MACE) up to three years. The ALSTER C.E.R.A. trial patient flow chart is presented in Figure 1.
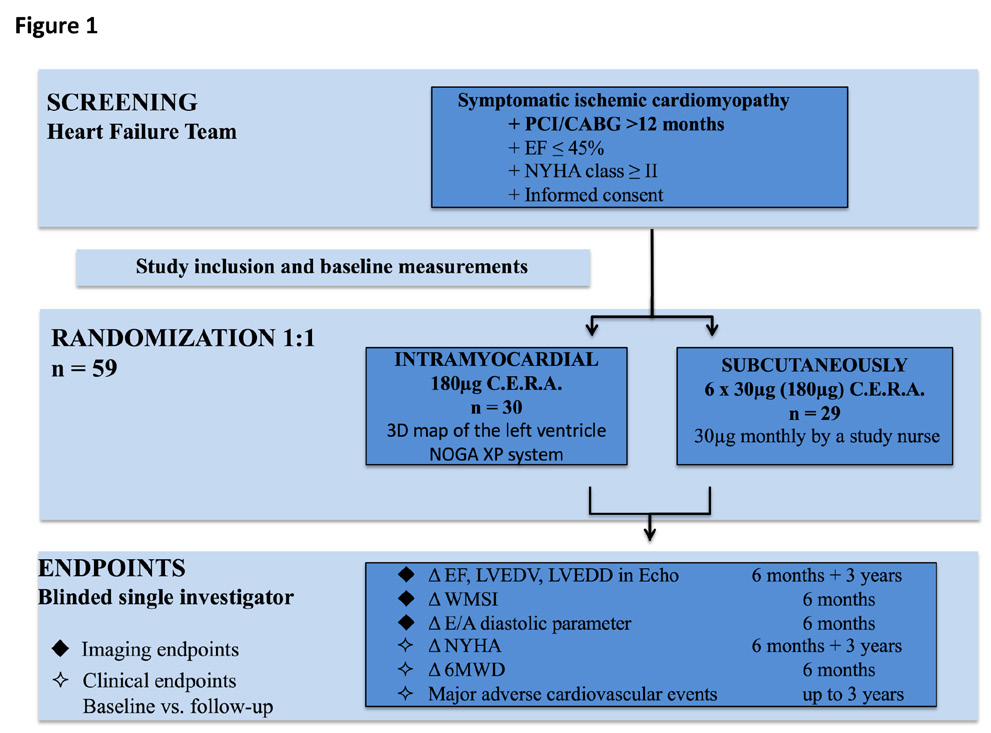
Figure 1 The ALSTER C.E.R.A. Trial flow chart.
CABG, coronary artery bypass grafting, E/A, diastolic parameter ratio, EF, ejection fraction, LVEDD, left ventricular end-diastolic diameter, LVEDV, left ventricular end-diastolic volume, MACE, major adverse cardiovascular events, PCI, percutaneous coronary intervention, WMSI, wall motion score index, 6MWD, 6-minute walking distance.
Treatment
Patients were randomly assigned to receive NOGA-guided intramyocardial injections or subcutaneous injections of C.E.R.A.. 3D left ventricular mapping was performed using the NOGA XP® system (BDS, Cordis, Johnson & Johnson, USA) only in patients randomized to the C.E.R.A. NOGA treatment group. The NOGA platform was proven to be safe and feasible in regenerative treatment with intramyocardial injections of stem cells in patients with ischemic heart disease.9 It allows precise placement of the injected substance to peri-ischemic regions (border zone) of the myocardium, leading to superior myocardial retention and reduces the likelihood of systemic effects. Every patient in the C.E.R.A. NOGA group received an electromechanical 3D LV map by point-by-point measurement. Usually > 100 verified points are necessary to obtain a complete LV map. The system distinguishes between viable (unipolar voltage > 12mV, bipolar voltage > 2.5mV, local linear shortening >6%), non-viable-myocardium (unipolar voltage < 6mV, bipolar voltage < 1.5mV, local linear shortening < 4%) and border zone (unipolar voltage 6-12mV, bipolar voltage 1.5-2.5mV, local linear shortening 4-6%) around myocardial scar tissue.10 Mismatch between unipolar and bipolar signals is observed when the myocardial scar is not transmural. Ischemic border zones were defined and marked after completion of the LV map; injections of C.E.R.A. (in total 180µg) into the border zone were performed with MYOSTAR® injection catheters (BDS, Cordis, Johnson & Johnson, USA) under 3D-guidance by the previously obtained LV electromechanical map. The number of injections varied, depending on the scar size and the decision to split the injected volume to cover all areas of the border zone, with adequate distance between the injection points. Exemplary images of the NOGA procedure are shown in Figure 2. Patients randomized to the subcutaneously application arm, received for six months subcutaneously C.E.R.A. injections of 30µg (180 µg in total), performed by a study nurse.
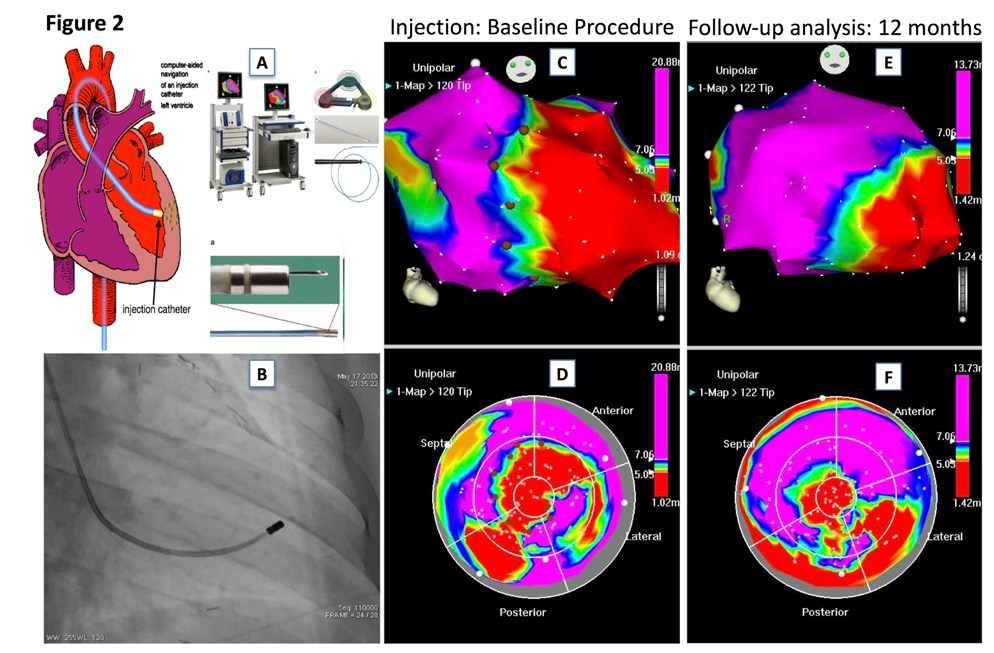
Figure 2 Exemplary images of a patient with ischemic heart failure and NOGA-guided intramyocardial injections of C.E.R.A.
2A. NOGA XP® system
2B. Fluoroscopic image of the MYOSTAR® injection catheter in the left ventricle
2C-D. 3D-left ventricular mapping after injection of C.E.R.A.; unipolar voltage in RAO (UPV 1-20mV) and bulls-eye projection (UPV with focus on the border zone 5-7mV) are displayed with margins manually set to standardized values. Colour encoding is displayed at the right upper border of the pictures. White points indicate mapping points, brown dots indicate locations for injections in the infarction border zone.
2E-F, A 3D-left-vetricular mapping after 12 months follow-up. Compare anterior apical scar area of the map in the bull’s eye perspective to the images 2C-D, suggestive of attenuated LV remodeling.
C.E.R.A.
C.E.R.A. (Continuous Erythropoietin Receptor Activator, MirC.E.R.A.®, Roche, Basel, Switzerland) has been developed for the treatment of anemia in patients with chronic kidney disease. C.E.R.A. has a unique pharmacological profile, that allows administration once monthly in comparison to the weekly administered epoetin β or darbepoetin α. Compared with epoetin-beta, C.E.R.A. has approximately 50- to 100-fold lower affinity for EPO receptor binding sites, mainly due to slower association. However, the different receptor-binding properties combined with a long half-life and slow systemic clearance of C.E.R.A. enable continuous stimulation of the tissue-specific EPO receptor.11
The dose of C.E.R.A. in patients with chronic kidney disease to maintain stable hemoglobin levels is 1.2 µg/kg body weight, applied once monthly subcutaneously (75-100 µg/month).12,13 Patients in this trial received low-dose C.E.R.A. subcutaneously (30 µg/month for 6 months) or 180 µg intramyocardial during the NOGA procedure, with multiple injections in the border zone. We chose this dose, based on the previous trial with low dose epoetin-beta, where patients received 35IU/kg body weight subcutaneously once monthly for 6 months 8 and the C.E.R.A. dosing instructions in equivalence to epoetin-beta (NeoRecormon®, Roche).
Cardiac imaging analysis
Echocardiography was performed at six- and 36-months follow-up according to current guidelines. Endocardial and epicardial borders were traced manually in end-diastole and end-systole and the mitral plane set to define the basal border of the LV, without using contrast. EF and LVEDV were calculated from the apical four- and two-chamber views using the biplane summation-of-discs method. LVEDD was measured in the parasternal longitudinal axis. WMSI was analyzed using a 16-segment model as previously described.14 With regard to reproducibility for every individual patient all exams were performed using the same echocardiography machine and performed by experts, blinded to the treatment.
Safety outcome assessment
During the three-year follow-up, serious adverse events were assessed. Analysis was based on individual patient level changes (follow-up vs. baseline) of echocardiographic parameters of LV remodeling (∆LVEDD and ∆LVEDV) as well as LV EF (∆EF). Clinical parameters (∆NYHA) were assessed at six months and three-years follow-up. Individual changes of the E/A-ratio (∆E/A), WMSI (∆WMSI) and 6MWD (∆6MWD) were measured between baseline and six months follow-up.
Statistical analysis
No formal sample size calculation was performed as the trial was conceived as exploratory. All analyzed subjects received the treatment to which they were randomly assigned and were retained in the group until endpoint. Statistical analysis was performed using GraphPad Prism version 8 (GraphPad Software, Inc., San Diego, CA, USA). Categorical data and MACE were analyzed by the Fisher Exact test. Predefined endpoints were the individual patient changes in EF, LVEDV, LVEDD, WMSI and E/A ratio as measured by echocardiography, as well as the NYHA class and the 6MWD between baseline and follow-up. Significance within one group was assessed by the Kolmogorov-Smirnov test, followed by the one-sample t-test or the Wilcoxon signed rank-test with “no change” (Δ=0) as the hypothetical (H0) value. Significance between the groups was assessed by analysis of the Mann-Whitney test. A p-value <0.05 was considered to be statistically significant. All results are reported as mean ± standard error of the mean.
Enrolment and baseline characteristics
60 patients were recruited to the study. One patient withdrew consent during the screening phase after providing informed consent. 30 patients were assigned to receive NOGA-guided injections of C.E.R.A. (C.E.R.A. NOGA group) and 29 to receive subcutaneously C.E.R.A. treatment (C.E.R.A. SC group). The two groups showed similar baseline characteristics (Table 1); by chance the C.E.R.A. NOGA group was retrospectively found to be significantly younger (C.E.R.A. NOGA 58.2±1.2 vs. C.E.R.A. SC 65.1±1.5 years, p=0.0013) and present with larger ventricles (LVEDD: C.E.R.A. NOGA 6.4±0.1 vs. C.E.R.A. SC 6.0±0.05cm, p=0.024; ∆LVEDV: C.E.R.A. NOGA 241.1±11.9 vs. C.E.R.A. SC 196.5±8.6ml, p=0.007) but similar baseline LV EF. Patients in both groups presented with symptomatic HF as judged by NYHA class. Patients were on stable maximal-tolerable HF medication, but no patient received an angiotensin receptor neprilisin inhibitor (ARNI) or sodium-glucose-cotransporter 2 (SGLT2) inhibitor treatment, as the patients were included in the trial prior to the release of the ESC heart failure guidelines 2016 and 2021 and follow-up was up to local standards. No patients were excluded due to cardiac resynchronization therapy implantation during the follow-up.
|
|
C.E.R.A. NOGA n=30 |
C.E.R.A. SC n=29 |
p-value |
|
mean±SEM |
|||
|
Gender male, n(%) |
28(93.3) |
28(96.5) |
>0.9(ns) |
|
Age, years |
58.2±1.2 |
65.1±1.5 |
0.0013(*) |
|
NYHA class |
2.9±0.04 |
2.9±0.06 |
0.42(ns) |
|
CABG, n(%) |
8(26.7) |
8(27.6) |
>0.9(ns) |
|
PCI, n(%) |
15(50) |
15(51.7) |
>0.9(ns) |
|
Atrial fibrillation, n(%) |
9(30) |
14(48.2) |
0.18(ns) |
|
EF, % |
27.3±1.3 |
29.2±1.1 |
0.36(ns) |
|
LVEDD, cm |
6.4±0.1 |
6.0±0.05 |
0.024(*) |
|
LVEDV, ml |
241.1±11.9 |
196.5±8.6 |
0.007(*) |
|
Medication, n(%) |
|||
|
ACE/AT1-inhibitors, |
26(86.7) |
25(86.2) |
>0.9(ns) |
|
Beta-blockers |
24(80) |
24(82.7) |
>0.9(ns) |
|
Diuretics |
17(56.6) |
20(68.9) |
0.4(ns) |
|
Aldosterone antagonists |
20(66.7) |
15(51.7) |
0.2(ns) |
Table 1 Baseline characteristics
CABG, coronary artery bypass grafting; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; PCI, percutaneous coronary intervention
Periprocedural feasibility, echocardiographic and clinical outcome parameters
We found NOGA-guided injections of C.E.R.A. to be feasible in this patient collective, without periprocedural or postprocedural serious adverse events. Clinical and echocardiographic findings are presented in Table 2 and Figures 3A-D. There was no specific correlation between the number of injections and the endpoints, since all patients received the same C.E.R.A. dose; split and precisely injected in the border zone, covering all areas.
|
|
C.E.R.A. NOGA n=30* |
C.E.R.A. SC n=29** |
p-value |
|
LVEDD, mean±SEM |
|||
|
Baseline LVEDD, cm |
6.4±0.11 |
6.0±0.05 |
0.024(*) |
|
DLVEDD 6 months vs. baseline, cm |
-0.16±0.1 p=0.09(ns) |
0.02±0.09 p=0.7(ns) |
0.1(ns) |
|
DLVEDD 3 years vs. baseline, cm |
0.02±0.1 p=0.8(ns) |
0.3±0.09 p=0.0026(*) |
0.06(ns) |
|
LVEDV, mean±SEM |
|||
|
Baseline LEDV, ml |
241.1±11.98 |
196.5±8.6 |
0.007(*) |
|
DLVEDV 6 months vs. baseline, ml |
-8±12.8 p=0.3(ns) |
8.13±7.5 p=0.2(ns) |
0.1(ns) |
|
DLVEDV 3 years vs. baseline, ml |
10±15.9 p=0.5(ns) |
34.8±11.3 p=0.0081(*) |
0.1(ns) |
|
EF, mean±SEM |
|||
|
Baseline EF, % |
27.3±1.3 |
29.2±1.1 |
0.3(ns) |
|
DEF 6 months vs. baseline, % |
4.2±1.2 p=0.0015(*) |
0.7±0.9 p=0.4(ns) |
0.0411(*) |
|
DEF 3 years vs. baseline, % |
2.4±1.2 p=0.045(*) |
-1.6±1.1 p=0.1(ns) |
0.0171(*) |
|
NYHA class, mean±SEM |
|||
|
Baseline NYHA class |
2.9±0.04 |
2.9±0.06 |
0.42(ns) |
|
DNYHA 6 months vs. baseline |
-0.6±0.11 p<0.0001(*) |
-0.2±0.09 p=0.07(ns) |
0.014(*) |
|
DNYHA 3 years vs. baseline |
-0.33±0.1 p=0.0352(*) |
-0.13±0.1 p=0.7(ns) |
0.1(ns) |
|
WMSI, mean±SEM |
|||
|
Baseline WMSI, |
36.1±0.8 |
37.2±0.7 |
0.2(ns) |
|
DWMSI 6 months vs. baseline |
-1.8±0.8 p=0.04(*) |
-1.1±0.7 p=0.1(ns) |
0.7(ns) |
|
E/A(ratio), mean±SEM |
|||
|
Baseline E/A |
1.1±0.08 |
1.6±0.15 |
0.0262(*) |
|
DE/A 6 months vs. baseline |
-0.13±0.06 p=0.05(ns) |
-0.05±0.17 p=0.8(ns) |
0.1(ns) |
|
6MWD(distance in m), mean±SEM |
|||
|
Baseline 6MWD |
255.7±6.7 |
260.7±8 |
0.6(ns) |
|
D6MWD 6 months vs. baseline |
+78.33±16.5 p<0.0001(*) |
+40.34±10.4 p=0.0003(*) |
0.1(ns) |
Table 2 Echocardiographic results and clinical outcome parameter
E/A, diastolic parameter ratio; EF, ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; ns: not significant; WMSI, wall motion score index; 6MWD: 6-minute walking distance
*FU at 3 years was only possible in 24 patients, because 6 patients died
** FU at 3 years was only possible in 20 patients, because 9 patients died
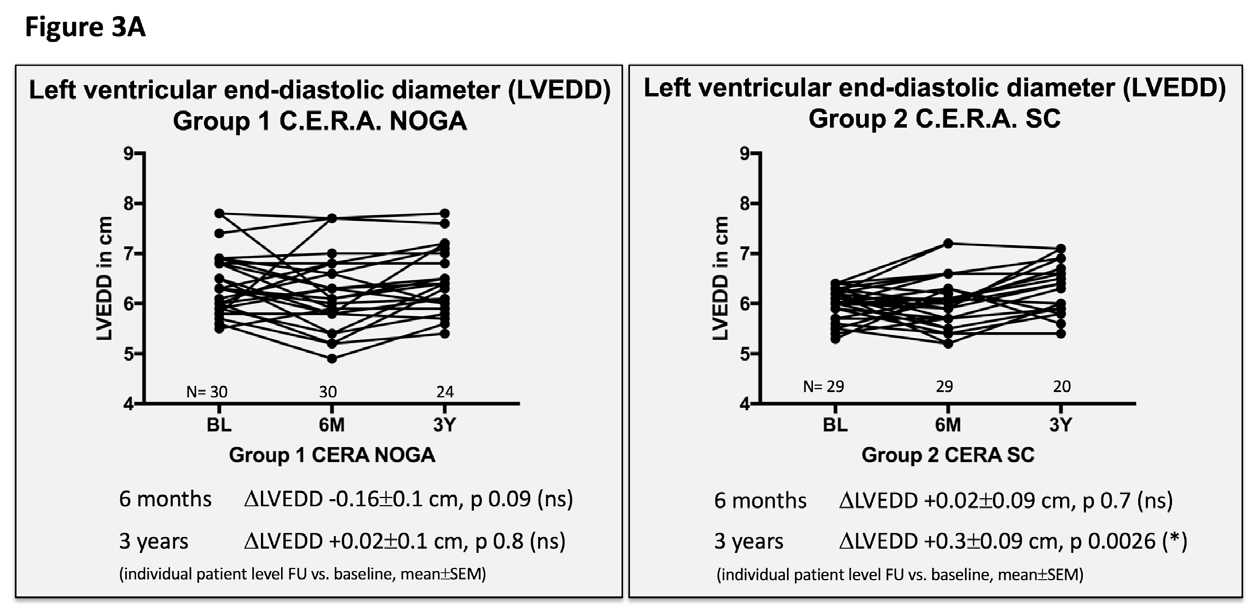
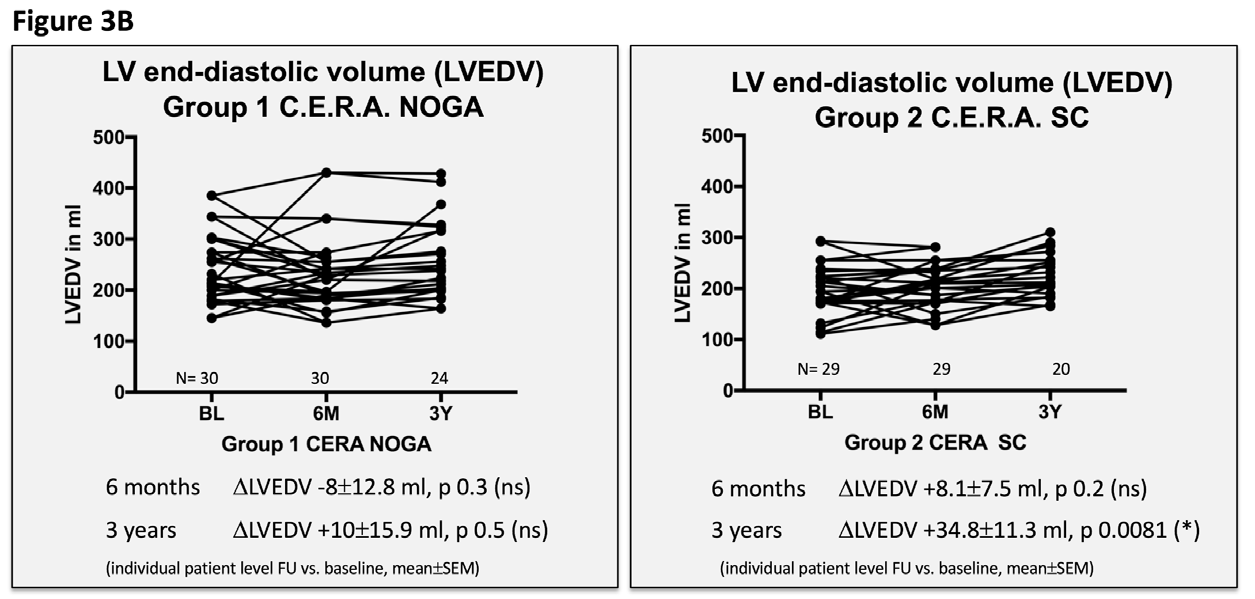
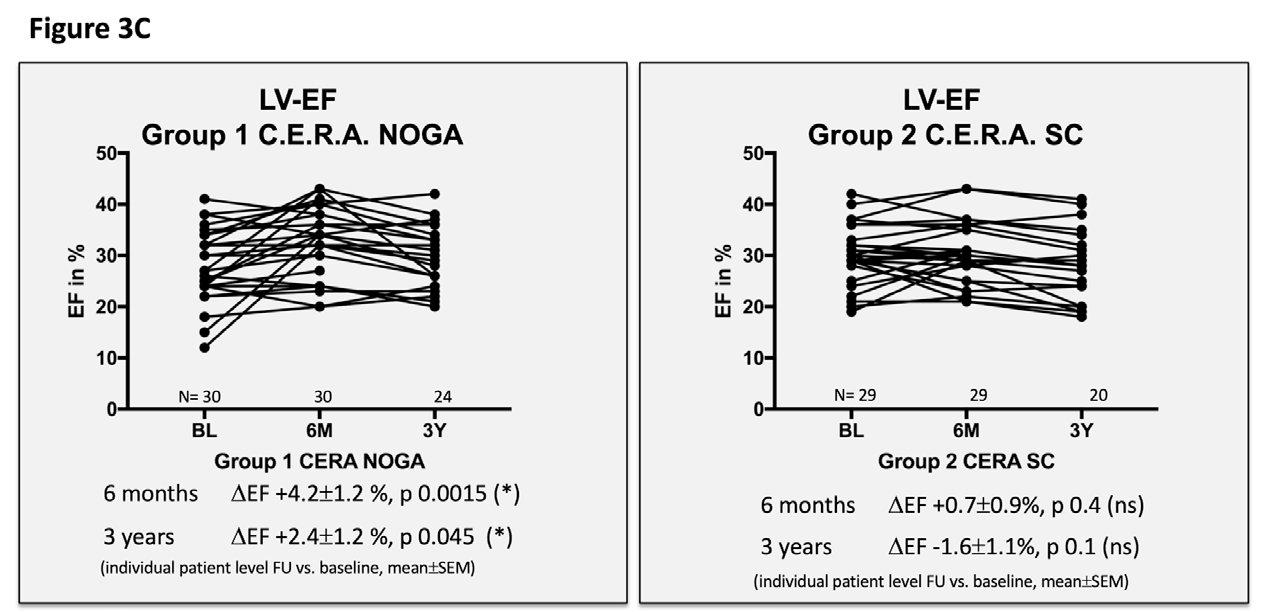
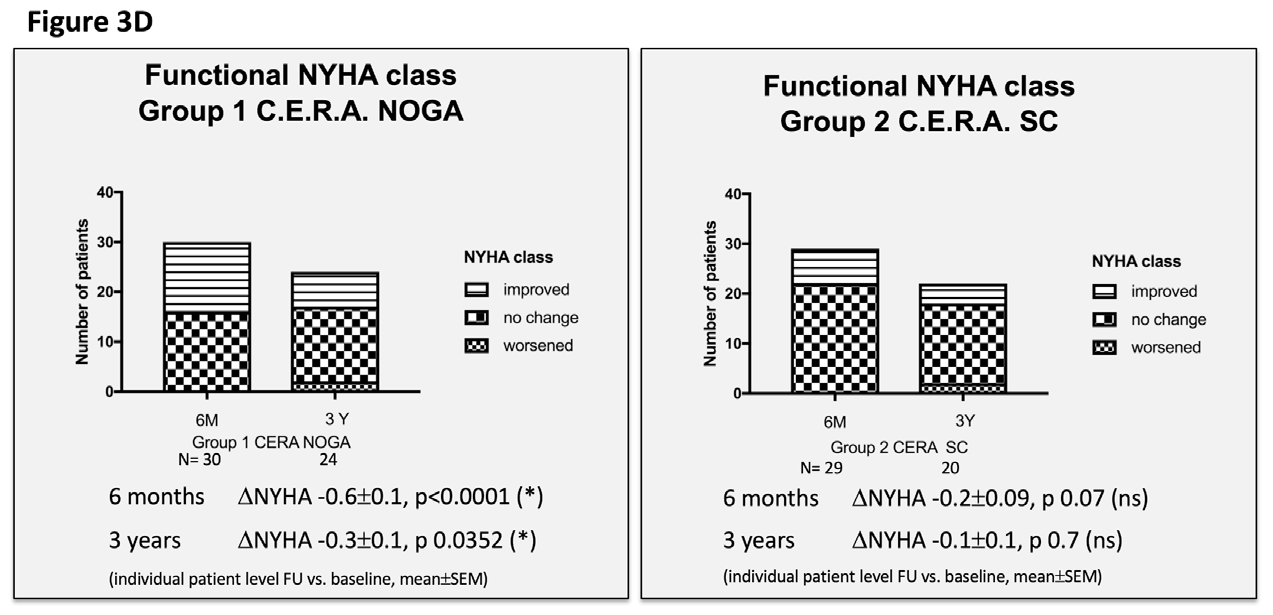
Figure 3 Echocardiographic and clinical results ALSTER C.E.R.A. trial; individual patient level follow-up at six months and three years for group 1 C.E.R.A. NOGA and group 2 C.E.R.A. SC for:
3A. Left ventricular end-diastolic diameter (LVEDD)
3B. Left ventricular end-diastolic volume (LVEDV)
3C. Left ventricular ejection fraction (LV EF)
3D. Functional NYHA class.
Patients in the C.E.R.A. NOGA group showed stable parameters for LVEDD and LVEDV after three years, while patients in the C.E.R.A. SC group had significant dilation of the LV (∆LVEDD: C.E.R.A. NOGA 0.02±0.1cm; p=0.8, C.E.R.A. SC 0.3±0.09cm; p=0.0026; ∆LVEDV: C.E.R.A. NOGA 10±15.9ml; p=0.5, C.E.R.A. SC: 34.8±11.3ml; p=0.0081). Changes in LVEDD and LVEDV were not statistically significant after six months for both groups. Furthermore, we observed an increase of the LV EF after six months (C.E.R.A. NOGA: ∆EF 4.2±1.2%; p=0.0015, C.E.R.A. SC: ∆EF 0.7±0.9%; p=0.4) and after three years in the C.E.R.A. NOGA group, while patients in the C.E.R.A. SC group presented with decrease in LV function (C.E.R.A. NOGA: ∆EF 2.4±1.2%; p=0.045, C.E.R.A. SC: ∆EF -1.6±1.1%; p=0.1). WMSI significantly improved after six months only in the C.E.R.A. NOGA patient group (∆WMSI C.E.R.A. NOGA: -1.8±0.8; p=0.04 vs. C.E.R.A. SC: -1.1±0.7; p=0.14) while we observed no change in the diastolic parameter E/A ratio in both groups.
We observed a significant improvement in the functional NYHA class, yet only in the C.E.R.A. NOGA group at six months (C.E.R.A. NOGA: -0.6±0.11; p<0.0001, C.E.R.A. SC: -0.2±0.09; p=0.7) and at three years (C.E.R.A. NOGA: -0.33±0.13; p 0.0352, C.E.R.A. SC: -0.13±0.1; p=0.7). The 6MWD distance significantly improved in both groups at six months; there was no assessment at three years follow-up.
Major adverse cardiovascular events
Data are presented in Table 3. We observed numerically more cardiovascular hospitalizations after three years in the C.E.R.A. SC group, but the difference was not significant. No stroke, myocardial infarction or stent thrombosis was observed during the three-years follow-up in both groups. Four elective percutaneous coronary intervention procedures in the C.E.R.A. NOGA and three in the C.E.R.A. SC group were performed due progression of coronary artery disease. There was no statistically difference in the number of anti-tachycardia pacing and implantable cardioverter defibrillator shock therapies between the groups. In addition, total mortality rates (Figure 4) at three years were comparable in both groups: six patients (20%) in the C.E.R.A. NOGA and nine patients (31%) in the C.E.R.A. SC group died during three-year follow-up.
|
|
C.E.R.A. NOGA n=30 |
C.E.R.A. SC n=29 |
p-value |
|
Mortality at 3 years, n(%) |
6(20) |
9(31) |
0.3(ns) |
|
CV hospitalization at 6 months, n patients with at least one hospitalization(%) |
4(13.3) |
6(20.7) |
0.5(ns) |
|
CV hospitalization at 3 years, n patients with at least one hospitalization(%) |
10(33.3) |
14(48.3) |
0.2(ns) |
|
Total number CV hospitalizations at 3 years |
13* |
22 ** |
0.14 |
|
Patients with more than one CV hospitalization at 3 years, n(%) |
2(6.6) |
7(24.1) |
0.06 |
|
Stroke at 3 years, n(%) |
0(0) |
0(0) |
>0.9(ns) |
|
Myocardial infarction at 3 years, n(%) |
0(0) |
0(0) |
>0.9(ns) |
|
ATP/shock of the ICD at 3 years, n(%) |
7(23.3) |
6(20.7) |
>0.9(ns) |
|
PCI at 3 years, n(%) |
4(13.3) |
3(10.3) |
>0.9(ns) |
Table 3 Major adverse cardiovascular events
ATP, antitachycardic pacing; CV, cardiovascular; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention
* Out of 10 patients with CV hospitalizations: n=1 with 2 events and n=1 with 3 events
** Out of 14 patients with CV hospitalizations: n=7 with 2 events
We evaluated the feasibility and safety of NOGA-guided intramyocardial injections of C.E.R.A. versus the subcutaneous application of C.E.R.A. in symptomatic ischemic HF patients with impaired LV function (EF ≤ 45%). We used a long acting erythropoietin analogue in this non-anemic patient collective in a dosing range that does not influence hemoglobin levels. Favorable pharmacological properties of C.E.R.A. enable continuous stimulation of the tissue-specific EPO receptor; they were the reason we chose this long acting EPO analogue for both treatment groups to achieve long term effects. No clinical study has tested the intramyocardial delivery route of a long-acting EPO-analogue (C.E.R.A.) in this patient collective to date. Intramyocardial delivery of C.E.R.A. was found to be feasible, safe and possibly able to attenuate LV remodeling in patients with ischemic cardiomyopathy.
The role of the erythropoiesis-independent effects of EPO remains poorly defined. Several experimental studies suggested cardio-protective effects of EPO independent of erythropoiesis via tissue-specific EPO receptors. EPO stimulates direct capillary outgrowth in an in vitro angiogenesis assay using adult human myocardial tissue.15 Systemic application of EPO prevented cardiac dilatation and improved cardiac function after myocardial infarction, by inducing mobilization and homing of endothelial progenitor cells from the bone marrow into the cardiac microvasculature and by increasing neovascularization in an animal model of myocardial infarction.3 Direct intramyocardial delivery of EPO has been investigated in several preclinical studies. A single intramyocardial high dose EPO administration increased capillary density in the peri-ischemic region and attenuated myocardial functional decline after myocardial infarction in rats.16 Transendocardial NOGA-guided delivery of EPO (versus placebo) showed an increase of mean unipolar voltage amplitude in the ischemic myocardial segments, a significantly reduced ischemic surface area, as well as reduced myocardial fibrosis by quantitative histological analysis in the EPO treated animals.17 It also improved LV-function and helped to obtain the myocardial contractile reserve, objectified by ultrasonic strain-rate imaging.18
Aside from the proangiogenic and antiapoptotic effects, EPO may enhance endogenous cardiac regeneration from local precursor cells. In a preclinical study, an EPO-receptor expression was described on cardiac progenitor cells isolated from adult mice. Furthermore, repetitive EPO administration induced cardiac progenitor cells differentiation towards cardiomyocyte-like cells, resulting in attenuation of LV remodeling after experimental myocardial infarction.1
So far, the applicability of these findings and the role of EPO in the clinical setting of cardiac repair remain unclear. Clinical studies investigating EPO after acute myocardial infarction show controversial results and to date no randomized trial could prove a reduction in infarct size; while some trials suggest safety of the method,6,19–21 or less major adverse cardiovascular events in the HEBE-III trial,5 others failed to show a benefit with even more cardiovascular events in the multicenter-randomized REVEAL trial.22 Of note, the administered EPO dose differed significantly in these non-anemic patients and administration was only performed during the index event. Patients with systolic HF and anaemia have worse symptoms, but so far causal relationship for worse outcome is not established.23 In the RED HF trial, treatment of anemia in HF patients with darbepoetin alfa, to achieve a hemoglobin target of 13g/dl, did not improve clinical outcome.24 However, a small pilot study found a sub-therapeutic dose of subcutaneously applied epoetin-b treatment following percutaneous coronary intervention for six months in patients with ischemic heart disease to be safe and effective by improving EF (analysed by high-resolution cardiac magnetic resonance imaging) and exercise capacity in a randomized, double-blind setting.8
In this study, patients with intramyocardial delivery of C.E.R.A. showed stable parameters for LVEDD and LVEDV over the three-year study period, while patients with subcutaneously application had a significant increase of LV diameter and volume as expected when LV remodeling occurs in ischemic cardiomyopathy. Furthermore, we observed a significant improvement of EF as measured by echocardiography after three years and improved clinical parameters. Cardiovascular hospitalizations were numerically reduced in the C.E.R.A. NOGA group, yet without reaching statistical significance and the mortality rate was not different at three years in this small study cohort.
A potential therapeutic applicability of these effects would be clarified by a carbamylated derivate of EPO (CEPO), an analogue that does not bind to the dimeric EPO receptor and lacks erythropoietic activity. CEPO showed similar cardio-protective effects like EPO in preclinical studies, without raising hemoglobin levels.25 CEPO is not clinically available so that local delivery of C.E.R.A. is currently the best option regarding tissue-specific effects of EPO independent from hematopoiesis. However, additional studies targeting the optimal erythropoietin-analogue C.E.R.A. dose with follow-up imaging evaluation by cardiac magnetic resonance imaging will be required before conclusions can be made regarding the efficacy of this approach.
The current study has limitations. We chose an open label design for practical and safety reasons. Our study is limited by its small sample size and variations in patient baseline data. Retrospectively, baseline differences in age, LVEDD and LVEDV were observed between the treatment groups. However, endpoint data were analyzed on individual patient level basis, comparing follow-up vs. baseline and patients with larger ventricles in the C.E.RA. NOGA group showed stable ventricle parameters after three years compared to patients in the C.E.R.A. SC group. No patient received an angiotensin receptor neprilisin inhibitor (ARNI) or sodium-glucose-cotransporter 2 (SGLT2) inhibitor treatment, as the patients were included in the trial prior to the release of the ESC heart failure guidelines 2016/2021. Therefore, the confounding factor of change in the HF treatment-strategy during follow-up was ruled out in this study. Echocardiographic measurements at three-year follow-up were only possible in 40 patients, due to the observed death events. Although we did not use an echo core lab, values were obtained in a blinded fashion. There was no predefined serial assessment of hemoglobin or hematocrit levels during follow-up. Of note, both non-anemic treatment groups received low dose C.E.R.A. (intramyocardial or subcutaneous) without effects on hemoglobin levels and laboratory examinations were performed routinely by the general practitioners, without reported adverse events regarding blood results.
In summary, intramyocardial administration of the long acting EPO analogue C.E.R.A. in ischemic cardiomyopathy is feasible and safe. Furthermore, EPO may be a therapeutic option to attenuate LV remodeling: first, experimental studies proved EPO receptors to be present on myocardial tissue-resident cardiac progenitor cells and EPO stimulation lead to cardiac progenitor cells differentiation towards cardiac-myocytes.1 Next, in a placebo-controlled study with EPO administered subcutaneously to patients with ischemic cardiomyopathy and EF ≥ 45% LV remodeling was improved.8 Now the long acting EPO analogue C.E.R.A. was found to possibly attenuate LV remodeling in ischemic cardiomyopathy with LV dysfunction (EF ≤ 45%) more potently if applied directly to the myocardium compared to the subcutaneous application over a study period of three years. While the evidence resides on small, single-center studies with patient numbers <100, the series of data warrants further larger studies to explore the mammalian endogenous repair system in hypoxia situations, namely EPO signaling, for the treatment of chronic ischemic cardiomyopathy.
None.
This research received no specific grant from any funding agency in the public, commercial, or not- for-profit sectors.
No conflicts to report.

©2022 Paitazoglou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.