Journal of
eISSN: 2373-4396


Research Article Volume 4 Issue 2
Department of Cardiology, Zagazig University Hospital, Egypt
Correspondence: Ragab Abdelsalam Mahfouz, Professor of cardiology, Algamah street- Zagazi University Hospital cardiology Department, Egypt, Tel 201006427671, Fax 20552357770
Received: October 30, 2015 | Published: December 4, 2015
Citation: Ragab A, Mahfouz, Amr A, Ateah, Elawady W, et al. (2015) Impact of Aortic Stiffness on Exercise Tolerance and Left Ventricular Filling Pressure after Percutaneous Coronary Intervention for Patients with Chronic Stable Coronary Artery Disease. J Cardiol Curr Res 4(2): 00137. DOI: 10.15406/jccr.2015.04.00137
Background and Aim: In patients with stable coronary artery disease aortic rigidity may contribute to exercise intolerance and impaired left ventricular (LV) function after coronary intervention. However, this relationship has not yet been investigated. We aimed to assess the impact of aortic stiffness on both exercise tolerance and left ventricular diastolic filling pressure after percutaneous coronary intervention (PCI).
Methods: A percutaneous coronary intervention and stenting was performed in 98 consecutive patients with stable coronary artery disease (CAD) and positive exercise ECG. Before and after PCI, echocardiographic study, exercise stress test and aortic stiffness indices were calculated.
Results: Aortic stiffness index was significantly higher in patients with CAD (19.5 ± 4.9vs 5.6 ± 2.1; P<0.0001), and still elevated even after successful PCI (11.5 ± 2.7; P<0.002). METs was significantly correlated with Aortic beta index (r = 0.64, P< 0.0001), LAVI (r= 0.49, P<0.001) and E/E′ (r =0.53, P <0.001). ASI of <15 and < 16.5 were the best cut-off values for prediction of improved exercise tolerance and LV filling pressure after PCI. The AUROC was calculated as 0.93 and 0.94 respectively (P<0.001).
Conclusion: In patients with stable coronary artery disease, increase in aortic rigidity, as assessed by aortic beta index, was independently correlated with reduced exercise tolerance and increased LV filling pressures, after PCI. ASI<15 and <16.5 were powerful predictors for improved exercise tolerance and decreased LV filling pressures after PCI.
Keywords: aortic stiffness, exercise tolerance, left ventricular filling, coronary stenting
ASI, aortic stiffness index; PCI, percutaneous coronary intervention; CAD, coronary artery disease; E/E, ratio of early mitral flow velocity wave (E) to the early mitral annular velocity wave (E’); LAD, left anterior descending artery; RCA, right coronary artery; LCX, circumflex artery; LAV, left atria volumes; BSA, body surface area; AOST, aortic strain
Aortic stiffness is associated with cardiovascular risk factors and atherosclerosis.1-3 It has been shown that aortic stiffness is a powerful predictor of future cardiovascular events and cardiovascular mortality.4 Several studies investigated the negative impact of aortic stiffness on coronary circulation. relationship between aortic stiffness and coronary circulation.5,6 Kingwell et al.7 demonstrated that aortic stiffness decreases the chest pain in patients with CAD. Fukuda et al.5 showed that pulse wave velocity (PWV) increased and was significantly correlated with the number of diseased vessels. Noninvasive evaluation is an important clinical issue for patients with suspected CAD. Despite the presence of various diagnostic tools, diagnosis of significant CAD may be a difficult clinical challenge. The prognostic role of aortic stiffness measurement in cardiovascular disease was demonstrated in previous studies.8-11
Considering the association between a stiff aorta and the rate percutaneous coronary intervention (PCI), the significance of aortic stiffness in prediction of exercise tolerance and left ventricular function after PCI is not well studied. We therefore sought to examine the impact of aortic stiffness on exercise tolerance and left ventricular filling after PCI for patients with stable angina pectoris.
Patient population
Ninty-eight patients with stable angina pectoris and 70 healthy subjects were included in the study, from Jan 2012 to September 2012 in zagazig university hospital. Inclusion criteria included patients presented with typical angina pectoris and had positive exercise stress test and with documented significant coronary arery disease ( coronary artery stenosis >70% of the artery lumen) in coronary angiography. Exclusion criteria included significant left main coronary artery disease, triple vessel disease, prior coronary artery bypass graft surgery, recent acute myocardial infarction (<6 wk), valvular heart disease, left ventricular ejection fraction <40%, atrial fibrillation, or other conduction disturbance or significant peripheral vascular disease. All subjects were informed about the study, and all gave written consent.
Echocardiographic asessment
Echocardiographic assessment was obtained utilizing an echo machine (GE-Vivid 3; General Electric, Milwaukee, WI, USA) with 2.5 and 3.5 MHz transducers. Harmonic imaging was activated and a frame-rate optimization (60-100 fps) was with adjusted for. Biplane Simpson disk method was used to calculate LV volumes and EF%.12 Diastolic function was assessed utilizing both conventional pulsed as well as pulsed tissue Doppler with the average of three cycles. The following parameters were obtained: peak velocities during systole (S) and early diastole (E′). The E/E′ ratio as an estimate of LV filling pressure.13 Left atrial (LA) volume in systole was also measured just before the mitral valve opening, using the biplane Simpson’s method, as a mean between the values recorded in apical four and two-chamber approaches. Subsequently, LAV was indexed for BSA, such as LAVI in mL/m2.14
Aortic stiffness measurement
The following aortic stiffness indices were calculated as previously described:15,16
Aortic strain (AOST) = systolic diameter (SD) – diastolic diameter (DD)/diastolic diameter (DD) \ 100.
Aortic distensibility = (2 \ aortic strain)/Pulse pressure (pure number); and elastic modulus E(p) = PP/strain.
Aortic stiffness index (beta) was calculated according to the following formula: (β) Index=ln (systolic blood pressure/diastolic blood pressure)/AOST.
Exercise test
Modified Bruce protocol utilizing Marquette T-2000 treadmill was applied for all patients. Electrocardiograms and blood pressure recordings were monitored throughout. Total exercise time, stage, maximum workload (metabolic equivalents, or METs), peak exercise, and resting heart rate were recorded.
Follow-up assessment
All patients underwent clinical follow-up as well as complete echo-Doppler study including ASI assessment and exercise stress test 3 months after the PCI.
Statistical analysis
SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used to analyse our results. Student’s t-test was used for comparison. Stepwise multiple linear regression analyses were performed. To calculate optimal cut off values the receiver operating characteristic (ROC) Analysis was performed. Sensitivity, specificity, predictive values, and accuracy were calculated by using typical formulas. The multivariate enter logistic regression was performed to identify the independent predictors of METs and E/E’ after PCI. Reproducibility of aortic stiffness index (ASI) was assessed in seven subjects. Biological variability (over 10 cardiac cycles) and inter observer variability (two observers selecting 10 cardiac cycles for analysis) had <8%.
PCI was successful in all a patients with a TIMI flow 3 was achieved. There were no major complications occur in hospital period and during the 3 months of follow-up. PCI was performed to Left Anterior Descending artery (LAD) in 45 patients, Circumflex artery (LCX) in 16 patients, Right Coronary Artery (RCA) in 24 patients and for both LAD and RCA in 13 patients. Baseline characteristics of the study groups are shown in (Table 1). The two groups were comparable with respect to age, body mass index, systolic and diastolic blood pressures, and heart rate. The mean ASI was 5.6 ± 2.1 in the controls versus 19.5 ± 4.9 in patients with CAD (p<0.001). Following successful PCI the mean ASI decreased significantly by 34.3% to 11.5 ± 2.7 (p<0.002). However, the post procedural ASI values were still significantly higher in patients than those in the control group (Table 2 and Figure 1).
|
Variables |
Patients (n = 98) |
Controls (n = 70) |
p |
|
Age (years) |
56.9 ± 5.2 |
54.6 ± 6.4 |
>0.05 |
|
Gender (males %) |
-70% |
-65% |
>0.05 |
|
Body mass index (kg/m2) |
25.8+3 |
24.7+5 |
>0.05 |
|
DM |
39 (38.7%) |
0 (0%) |
- |
|
Hypertension |
48 (49%) |
0 (0%) |
- |
|
Smoking |
35 (36%) |
0 (0%) |
- |
|
SBP |
139 ± 11.5 |
136.5 ± 9.3 |
>0.05 |
|
DBP |
83.5 ± 4.5 |
84.2 ± 3.9 |
>0.05 |
|
EF% |
65.3 ± 6.4 |
67.2 ± 5.7 |
0.26 |
|
FS% |
34.3 ± 3.2 |
35.25 ± 4.1 |
0.34 |
|
LAVI mL/m2 |
37.6 ± 5.4 |
21.5 ± 4.2 |
<0.001 |
|
E |
68.1 ± 12.1 |
76.3 ± 6 |
>0.05 |
|
A |
58.9 ± 9.1 |
56.5 ± 3.3 |
>0.05 |
|
E/A |
1.14 ± 0.41 |
1.24 ± 0.2 |
>0.05 |
|
E′ mean (m/s) |
6.1 ± 1.4 |
11.3 ± 1.7 |
<0.05 |
|
E/E” |
14.3±0.11 |
6.1±0.11 |
< 0.001 |
|
S mean (cm/s) |
0.08+0.02 |
0.14+0.03 |
>0.05 |
|
AOST % |
8.7 + 2.3 |
14.8 + 2.8 |
<0.001 |
|
Distensibility, mmHg1 .103 |
3.3 ± 1.3 |
4.9 ± 1.5 |
<0.01 |
|
Elastic modulus N/m2 |
6.9 ± 2.8 |
4.1 ± 1.7 |
<0.01 |
|
Aortic Stiffness Index |
19.5±4.9 |
5.6 |
<0.001 |
Table 1 Demographic and Echocardiographic characteristics of patients versus control subjects
DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; FS, fractional shortening; LAVI, left atrial volume index; E, E-wave velocity; A, A-wave velocity AOST, aortic strain
|
Variables |
Before PCI |
3 Months After PCI |
P |
|
Stress test |
|||
|
- Negative |
0 |
85 (86.7%) |
<0.001 |
|
- Positive |
98 |
13 (13.3%) |
|
|
Duration of exercise (minutes) |
7.85±1.02 |
12.16±1.53 |
< 0.001 |
|
METs |
7.87 ± 1.75 |
10.65 ± 1.6 |
< 0.001 |
|
LVEDD |
47.25 ± 5.7 |
48.30 ± 5.2 |
0.07 |
|
LVESD |
31.4 ± 4.2 |
31.1 ± 7.4 |
0.76 |
|
EF |
65.2 ± 6.3 |
66.4 ± 7.1 |
0.06 |
|
E/A |
1.05 ± 0.4 |
1.28 ± 0.3 |
< 0.001 |
|
LAVI mL/m2 |
37.6 ± 5.4 |
29.5+3.2 |
< 0.01 |
|
E / E ' mean |
14.3±0.11 |
8.6±0.09 |
< 0.01 |
|
S mean, m/s |
0.08+0.02 |
0.11+0.04 |
>0.05 |
|
ASI |
19.5 ± 4.9 |
11.5 ± 2.7 |
< 0.001 |
Table 2 Exercise and chocardiographic parameters before PCI and 3 months after PCI
METs, metabolic equivalent; LAVI, left atrial volume index; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; EF%, ejection fraction; E/E, ratio of early mitral flow velocity to myocardial early velocity; ASI, aortic stiffness index
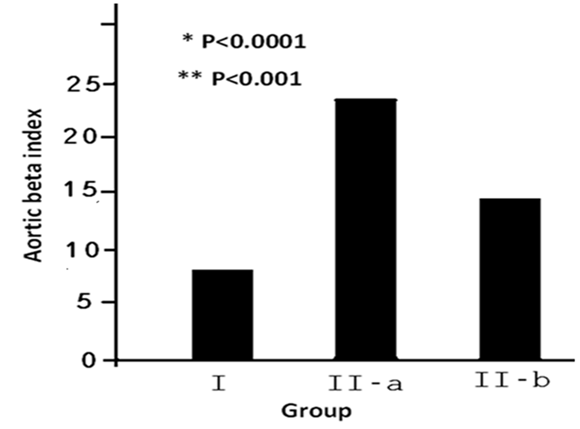
{*: the difference patients before PCI (IIa) and control subjects (I). While**: the difference patients after PCI (IIb) and control subjects (I)}.
Figure 1 Aortic stiffness Index before and after PCI in patients with stable CAD [before PCI (IIa) & after PCI (IIb) versus control subjects (I).
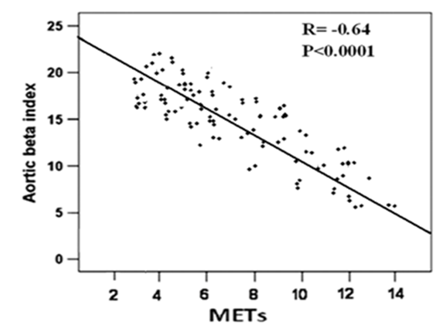
Figure 2 Linear regression analysis and Pearson correlation coefficient for the relationship between exercise capacity (METs) and aortic beta index.
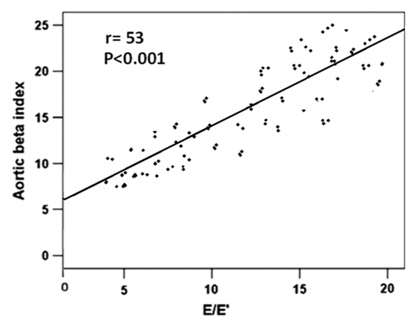
Figure 3 Linear regression analysis and Pearson correlation coefficient for the relationship between E/E′ and aortic beta index.
Thirteen patients had positive stress test and a significantly elevated LAVI (P<0.03) and significantly elevated LV filling pressure (P<0.01). ASI was significantly higher in those with positive than in patients with negative exercise test after PCI {AOST %:7.9 + 2.5vs 11.6 + 3.7 (P<0.001), Distensibility, mmHg1.103: 2.9 ± 1.5vs 4.8 ± 1.2 (P <0.01); Elastic modulus N/m2: 8.3 ± 1.5vs 5.8 ± 1.7 (P<0.001) and ASI: 23.9+ 2.8.8vs 13.5+1.1 (P <0.001)} (Table 3).
|
Patients with Negative Stress Test after PCI (n=85) |
Patients with Positive Stress Test after PCI (n=13) |
P-Value |
|
|
LVEF, % |
63+6 |
62+6 |
>0.05 |
|
LAVI mL/m2 |
26.1+2.7 |
33.5+ 3.4 |
< 0.03 |
|
E / A |
1.29+ 0.04 |
1.21+0.03 |
>0.05 |
|
S mean, m/s |
0.12+0.02 |
0.10+ 0.02 |
>0.05 |
|
E / E' mean |
5.98 +1.9 |
10.83+ 2.1 |
<0.01 |
|
Aortic stiffness Indices |
|||
|
AOST % |
11.6 + 3.7 |
7.9 + 2.5 |
<0.001 |
|
Distensibility (mmHg1 .103) |
4.8 ± 1.2 |
2.9 ± 1.5 |
<0.01 |
|
Elastic modulus (N/m2) |
5.8 ± 1.7 |
8.3 ± 1.5 |
<0.01 |
|
Aortic stiffness index (β) |
13.5+1.1 |
23.9+ 2.8 |
<0.001 |
Table 3 Echocardiographic indices of patients with negative Stress test versus those with positive Stress test after PCI
LVEF, left ventricular ejection fraction; FS, fractional shortening; LAVI, left atrial volume index, AOST, aortic strain
Correlation analysis before and after PCI, showed that METs was inversely correlated with age (-r= 0.31, P<0.05; -r = 0.28, P < 0.05), peak S (P < 0.02), E’ mean (P <0.01 &<0.03), LAVI (P< 0.01 & <0.001) , E/E′ ratio (-r = 0.55 & 0.53, P < 0.001), and ASI (r =0.61& 0.64, P < 0.001) (Table 4). On the other hand E/E’ was significantly correlated with LAVI ml/m2 (r=0.58& 0.62; P<0.001), and aortic beta index (r =0.61& 0.64, P < 0.001), (Table 5). With multiple regression analysis, ASI emerged to be the strongest independent predictor of improved exercise indices and decreased E/E’ after PCI (Table 6 & 7).
|
|
Before PCI |
After PCI |
||
|
Variable |
r |
P-value |
r |
P-value |
|
Age, y |
-0.31 |
<0.04 |
-0.28 |
<0.05 |
|
BMI |
-0.22 |
>0.05 |
-0.19 |
>0.05 |
|
LVEF, % |
0.26 |
>0.05 |
0.21 |
>0.05 |
|
LAVI mL/m2 |
-0.45 |
<0.01 |
-0.49 |
<0.001 |
|
S mean m/s |
0.43 |
<0.02 |
0.42 |
<0.01 |
|
E/A ratio |
-0.21 |
>0.05 |
-0.23 |
>0.05 |
|
E′'mean |
-0.44 |
<0.02 |
-0.41 |
<0.03 |
|
E/E’ |
-0.55 |
<0.001 |
-0.53 |
<0.001 |
|
ASI |
-0.61 |
<0.0001 |
-0.64 |
<0.0001 |
Table 4 Linear Regression Analysis (Univariate Analysis) for prediction of METs before and after percutaneous coronary intervention
BMI, body mass index; LVEF, left ventricular ejection fraction; LAVI, left atrial volume index; ASI, aortic stiffness index
|
|
Before PCI |
After PCI |
||
|
Variable |
r |
P-value |
r |
P-value |
|
Age, y |
-0.31 |
<0.04 |
-0.28 |
<0.05 |
|
BMI |
-0.22 |
>0.05 |
-0.19 |
>0.05 |
|
LVEF, % |
0.26 |
>0.05 |
0.21 |
>0.05 |
|
LAVI mL/m2 |
-0.45 |
<0.01 |
-0.49 |
<0.001 |
|
S mean m/s |
0.43 |
<0.02 |
0.42 |
<0.01 |
|
E/A ratio |
-0.21 |
>0.05 |
-0.23 |
>0.05 |
|
E′'mean |
-0.44 |
<0.02 |
-0.41 |
<0.03 |
|
E/E’ |
-0.55 |
<0.001 |
-0.53 |
<0.001 |
|
ASI |
-0.61 |
<0.0001 |
-0.64 |
<0.0001 |
Table 5 Linear Regression Analysis (Univariate Analysis) for prediction of E/E' before and after percutaneous coronary intervention
BMI, body mass index; LVEF, left ventricular ejection fraction; LAVI, left atrial volume index; ASI, aortic stiffness index
|
Variable |
OR |
95% CI |
P value |
|
Age (year) |
1.8 |
0.5-4.8 |
>0.05 |
|
Body mass index (kg/m2) |
1.5 |
0.3-4.1 |
>0.05 |
|
SBP (mmHg) |
2.7 |
1.1-6.9 |
<0.05 |
|
DBP (mmHg) |
1,6 |
0.4-4.3 |
>0.05 |
|
LAVI (mL/m2) |
3.7 |
1.3-8.8 |
<0.01 |
|
LV EF% |
1.9 |
0.5-4.5 |
>0.05 |
|
ASI |
6.5 |
2.5-13.5 |
<0.001 |
Table 6 Multivariate logistic regression analysis for prediction of improved exercise tolerance
SBP, systolic blood pressure; DBP, diastolic blood pressure; LAVI, left atrial volume index; EF%, ejection fraction; ASI, aortic stiffness index
|
Variable |
OR |
95% CI |
P value |
|
Age (year) |
1.65 |
0.48-4.50 |
>0.05 |
|
Body mass index (kg/m2) |
1.59 |
0.36-4.31 |
>0.05 |
|
SBP (mmHg) |
3.2 |
1.82-7.46 |
<0.05 |
|
DBP (mmHg) |
1.9 |
0.55-4.35 |
>0.05 |
|
LAVI (mL/m2) |
4.3 |
1.75-9.22 |
<0.02 |
|
LV EF% |
1.82 |
0.52-4.33 |
>0.05 |
|
ASI |
7.35 |
2.5-14.8 |
<0.001 |
Table 7 Multivariate logistic regression analysis for prediction of decreased LV filling pressure
SBP, systolic blood pressure; DBP, diastolic blood pressure; LAVI, left atrial volume Index; EF%, ejection fraction; ASI, aortic stiffness index
Receiver operating characteristic curve analysis showed that ASI <15 was the best cut off value for the prediction of improved exercise tolerance (increased METs), with a sensitivity, specificity, positive predictive value and negative predictive value of (95%, 64%, 94% and 70% respectively). The area under the curve was 0.93 (0.873-0.985) (Figure 4). While the best cut-off value in predicting decreased LV filling pressure (significantly decreased E/E’) was <16.5, with a sensitivity, specificity, positive predictive value and negative predictive value of (96%, 63%, 95% and 68% respectively). The AUROC was calculated as 0.94 (0.872–0.992) (Figure 5).
We demonstrated that aortic stiffness was inversely correlated with exercise tolerance and directly correlated with LV filling pressure (E/E’) in subjects with stable ischemic heart disease before and after PCI. Moreover the decrease in ASI significantly correlated with improved exercise tolerance and LV diastolic function after PCI for subjects with patients with chronic CAD. We have been demonstrated that ASI of <15 and < 16.5 were the best cut-off values for prediction of improved exercise tolerance and decreased LV filling pressure (E/E’) after PCI. Compared to healthy subjects, we found that patients with significant coronary lesions had significantly higher ASI values, even after successful PCI. We observed that ASI values significantly decreased after PCI. The exact mechanism for the decrease in ASI after PCI is uncertain. Successful PCI have been associated with hemodynamic and biochemical effects in the early term, especially improvements in left ventricular function and neurohumoral activation.17-18 These changes after PCI may be responsible for ASI changes after PCI.
Significant correlations have been found between ASI and parameters of left ventricular diastolic and systolic functions.16-20 These changes after PCI may be responsible for PWV changes in the early term.
Enko K et al.21 had demonstrated that, patients with high PWV had lower exercise capacity than patients with low PWV. A low myocardial ischemia threshold, as well as an enhancement of the ventilatory response to exercise, was also found in patients with high PWV. The peripheral arteris are less stiffening than asscenidin aorta and consequantly don’t reflect the exact load imposed by vascular system on left ventricle.22,23 In addition stifness in ascending aorta can be assessed easly and noninvasively with mor accuracy tha peripheral arteries.24
Stiffer central vessels may affect V̇o2, influencing LV relaxation. The association between a higher PWV and a more restrictive mitral filling pattern is consistent with this. The association between a higher PWV and a more restrictive mitral filling pattern is consistent with this. A link between greater aortic stiffness and a restrictive mitral filling pattern could occur as a result of cardiac hypertrophy, changing the properties of both the myocytes and the interstitium, which is known to be a factor that influences LV diastolic filling.25,26
Clinical implication
Changes in ASI may be a clinically important parameter in predicting exercise response and may become an additional target for therapeutic interventions that aim to improve quality of life and ventricular burden of subjects with CAD.
The current study demonstrated that aortic stiffness is an independent predictor exercise tolerance and left ventricular filling pressures before and after PCI. Our data provided that an ASI < 15 and < 16.5 were the best cut-off values for prediction of improved exercise tolerance and reduced LV filling pressure respectively after successful PCI.

©2015 Ragab, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.