Journal of
eISSN: 2373-4396


Clinical Images Volume 17 Issue 1
1Department of Radiodiagnosis and Imaging, Postgraduate Institute of Medical education and Research, Chandigarh, India
2Department of Cardiology, Advanced Cardiac Centre, Postgraduate Institute of Medical education and Research, Chandigarh, India
Correspondence: Dr. Arun Sharma, MD, DM, Department of Radiodiagnosis and Imaging, PGIMER, Chandigarh, India, Tel +91 9910380907
Received: December 27, 2023 | Published: February 5, 2024
Citation: Singhal M, Shantagiri A, Bhasin D, et al. Hypertrophic cardiomyopathy with obstructive coronary artery disease: Importance of identifying distinct LGE patterns. J Cardiol Curr Res. 2024;17(1):4-5. DOI: 10.15406/jccr.2024.17.00596
Late gadolinium enhancement (LGE) in hypertrophic cardiomyopathy (HCM) is usually of patchy midmyocardial pattern at the right ventricular insertion site within the hypertrophied myocardium and suggests fibrosis.1 LGE in an ischemic event is usually subendocardial and territorial. Knowledge of the proper history and the imaging correlation is needed to accurately interpret cardiac magnetic resonance (CMR) imaging as few cases may have LGE (replacement fibrosis) due to HCM with a concomitant ischemic territorial subendocardial fibrosis as seen in the index case.
A 64-year-old man with no prior cardiac illness presented with typical angina with ST-T wave changes on electrocardiograph (ECG) and elevated cardiac troponins requiring emergent catheter angiogram which showed 95% stenosis in the mid left anterior descending artery (LAD) for which percutaneous coronary intervention was done (Figure 1). The ECG and 2D-transthoracic echocardiogram (TTE) also showed changes of asymmetric left ventricular hypertrophy with a significant outflow tract gradient consistent with obstructive HCM. As a part of the workup for HCM, the patient underwent a CMR imaging that revealed asymmetric thickened basal and mid septum (22 mm in end diastole), with relatively thinned out and hypokinetic left ventricular (LV) apex. There was subendocardial LGE along the LAD territory with midmyocardial enhancement involving the hypertrophied septum (Figure 2).
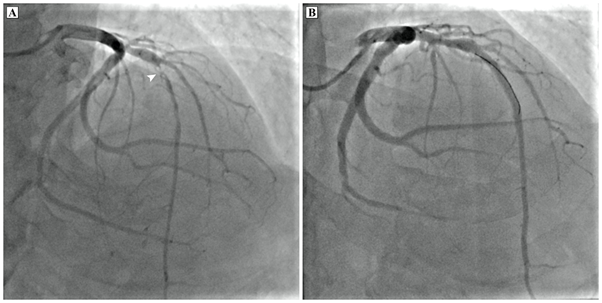
Figure 1 Coronary angiogram of the left coronary system in cranial right anterior oblique projection showing ~95% stenosis in the mid segment of left anterior descending artery (arrowhead). (B) Coronary angiogram of the left coronary system in cranial right anterior oblique projection after successful stenting of mid left anterior descending artery with a 3.5 x 38 mm drug eluting stent. A coronary wire is seen in situ.

Figure 2 Cardiac MRI (a) steady state free precision diastolic short axis image demonstrating asymmetric septal predominant hypertrophic cardiomyopathy (thick arrows). (b) Horizontal long axis and (c) short axis late gadolinium enhancement images show subendocardial late gadolinium enhancement (thin arrows) with thinning of apex which showed hypokinesis on cine imaging and midmyocardial patchy fibrosis in thickened septum (thick arrows).
HCM is a hereditary cardiac disorder with myocardial fibrosis as the major histological feature.2,3 LGE at CMR is the reference standard for non-invasive identification of myocardial replacement fibrosis.4,5 Moreover, distinct LGE patterns are seen in patients with HCM and coexisting coronary artery disease.2 Subendocardial LGE in a particular coronary territory in a patient with HCM suggests prior myocardial infarction because of coronary artery disease, while intramyocardial patchy LGE is a marker of replacement fibrosis secondary to HCM per se. In patients with the territorial infract/fibrosis, coronary evaluation should be considered if not previously done. Identification of coronary artery disease is important as these patients should receive drugs for secondary prevention of coronary artery disease such as aspirin and statins in addition to medical therapy for HCM. Another cause of such pattern can be prior myocardial infarction secondary to coronary embolism. The source of embolism in patients with HCM may be an associated apical aneurysm or due to atrial fibrillation.6 Moreover, the apical variant of HCM may show “MI pattern” of LGE without epicardial coronary involvement.7,8 The distinction is also important because of prognostic implications. The present case highlights the importance of identifying distinct LGE patterns in such patients.
Authors declare no conflicts of interest.
Authors confirm that the manuscript is solely submitted to the journal and not submitted/ being considered anywhere else.

©2024 Singhal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.