Journal of
eISSN: 2373-4396


Research Article Volume 14 Issue 4
1Department of Pathology, Universidade Federal de São Paulo, Brazil
2Department of Medicine, Universidade Federal de São Paulo, Brazil
3Nephrology Department, A Beneficência Portuguesa de São Paulo, Brazil
4Intensive Care Unit, A Beneficência Portuguesa de São Paulo, Brazil
Correspondence: Tania Leme da Rocha Martinez, BP - A Beneficência Portuguesa de São Paulo Rua Comandante Ismael Guilherme, 358 - Jardim Lusitânia, CEP 04031-120 - São Paulo – SP, Brazil, Tel 55 11 98323-9863, Fax 55 11 3842-3789
Received: July 18, 2021 | Published: August 17, 2021
Citation: Pomaro DR, Fonseca FAH, Saldanha ALR, et al. Diabetes and hypercholesterolemia experimental study on inhibition of liver fibrosis by angiotensin converting enzymes. J Cardiol Curr Res. 2021;14(4):107-112. DOI: 10.15406/jccr.2021.14.00524
Purpose: The aim of this study was to evaluate the effect of angiotensin-converting enzyme (ACE) inhibitor on the liver histopathological changes in hypercholesterolemic and diabetic rabbits.
Methods: New Zealand rabbits were treated by a single dose of alloxan (100mg iv) and were fed a chow with 0.5% cholesterol for 12weeks. The animals were divided into four groups according to the level of blood glucose: ≥250 mg/dL for groups I (n =10) and II (n=8) or <250mg/dL for groups III (n=12) and IV (n=12) and the groups II and IV were treated with an ACE inhibitor, quinapril (30mg/d) added to the diet. Total serum cholesterol and glucose levels and ACE activity were determined. Histological analysis was performed on liver samples stained with hematoxylin and eosin and picrosirius red. Liver fibrosis was analyzed by Metavir classification and was quantified by Image Tool software in picrosirius polarized images.
Results: The four groups were hypercholesterolemic, without significant statistical differences in cholesterol levels among them. ACE activity was lower in the plasma of animals treated with ACE inhibitor (groups II and IV, p<0,01). We observed lower collagen area determined histomorphometrically in the both groups treated with ACE inhibitors, although only in the group with blood glucose control, there have been statistically significant. (p<0.05; group IV<III).
Conclusion: Our results suggest a benefit in liver protection achieved by the use of an ACE inhibitor in hypercholesterolemic and diabetic rabbits. This study highlights the importance of the renin-angiotensin system in protecting organs affected by high levels of lipids and glucose.
Keywords: liver fibrosis, ace inhibitor, rabbit, diabetes, cholesterol
ACE, angiotensin-converting enzyme; ACEI, angiotensin-converting enzyme inhibitors; AT1, angiotensin II type 1 receptor; HSC, hepatic stellate cell; RAS, renin-angiotensin system
In experimental studies with hypercholesterolemic rabbits, besides the development of atherosclerosis, the high-cholesterol diet leads to a series of hepatocellular modifications, with lipid accumulation in parenchymal cells (steatosis), inflammatory reaction and even fibrosis. The fibrosis in the liver may be present as a central portal and lobular diffuse aspects and eventually as cirrhosis.1–4
Marked hypercholesterolemia induced in rabbits by diet is resulted from the absorption of large amounts of dietary cholesterol without increasing compensatory degradation and excretion of cholesterol. Rabbits fed cholesterol-rich foods for a long period of time develop portal fibrosis or cirrhosis in liver. There was diffuse fibrosis resulting in disorganization of lobular architecture, observing initially, liver lipid changes in the central lobular and accumulation of cholesterol in the liver parenchyma. Rabbits maintained on a hypercholesterolemic diet for different periods develop central lobular liver fibrosis in varying degrees.1 This fibrosis is characterized by deposition of collagen types I, III and IV, and the presence of macrophages and alpha-actin positive cells, indicate activation of fat storing cells.
Many studies have shown that HSC and other cells of myofibroblast lineage play a central role in liver fibrosis development.5,6 However, the mechanism of activation of these cells and fibrogenic response in the liver remains incompletely understood. The HSC is the predominant fibrogenic cell type in the liver. In normal liver, HSC are quiescent and following injury, they become activated and transform into interstitial myofibroblasts that are capable of producing the extracellular matrix components of fibrotic tissue, as well as a broad array of profibrotic and pro-inflammatory cytokines and chemokines.
Drugs therapy targeting the Renin-Angiotensin System (RAS) inhibiting the Ang II formation (Angiotensin Convertin Enzyme inhibitors - ACEI) or the Ang II binding to its receptor (Angiotensin Receptor Blockers) are now widespread in clinical use and have been shown to reduce tissue injury independently of their effects on blood pressure. Apart from the circulating RAS, the existence of local or intra-organ RASs have been described in a number of organs, including the heart, kidney, lung, pancreas and liver.7–9 The importance of the RAS in hepatic fibrosis also has been evidenced by studies in animals and humans, in whom treatment with ACEI and Angiotensin II type 1 receptor (AT1) blockers had beneficial effects on disease liver.10,11
Angiotensin II is present in both plasma and liver tissue from normal animals and is increased significantly in rat models of liver disease12 and cirrhotic patients.13 There is many evidence that angiotensin II, the main effector peptide of the RAS, is involved in the recruitment of inflammatory cells and transformation of HSC into an activated phenotype,7 indicating that RAS is involved in the pathophysiology of hepatic fibrosis.14,15 The Angiotensin II acts as a mediator of inflammation and fibrosis in chronic liver, activating the HSC, infiltration of inflammatory cells, and extracellular matrix protein synthesis.7,16,17
In the fibrotic liver, angiotensin-converting enzyme (ACE) and AT1 receptor protein expression is also localized to fibrous septa, mesenchymal cells (HSCs and myofibroblasts) and Kupffer cells.18–20 Studies with animal models have demonstrated that angiotensin II-blocking agents, such as ACEI and angiotensin II type 1 receptor antagonist, attenuate liver fibrosis.21–24 Numerous studies using a variety of animal models have demonstrated antifibrotic effect of the RAS inhibitor drugs.23,25–28
Studies suggest that Ang II could mediate and exacerbate liver fibrosis through the activation of cells stellate and the stimulation of transforming growth factor-β1 (TGFβ-1).29 Ang II induces contraction and proliferation of HSC, considered the main effector of hepatic fibrosis. The AT1 receptors are present in most mesenchymal cells and mediate most biological effects of Ang II, including increased intracellular calcium, and contraction cell proliferation.30 The intensity of the contractile response to Ang II by cells hepatic stellate was comparable to that observed after stimulation of these cells with endothelin 1, considered the most powerful contractile agent for this cell line.
The inhibition of the RAS reduces the expression of collagen type IV and expansion interstitial in different tissues. Some studies showed reduced messenger RNA levels of TGF-β 1 and procollagen I in the livers of rats treated with captopril after duct ligation common bile duct, supporting the hypothesis of an action of Ang II in cells hepatic stellate.24,30
Many studies have suggested a possible relationship between the RAS and the pathogenesis of insulin resistance, and the angiotensin-II may be a mediator of fibrosis in diabetic liver. The aim of this study was to evaluate the effect of ACEI on the liver fibrosis in hypercholesterolemic and diabetic rabbits.
Animals and experimental design
All the experimental procedures were conducted according to the National Institute of Health guidelines for the use and care of animals, and the study protocol was approved by the Ethics in Research Committee of the Federal University of São Paulo. New Zealand white male rabbits weighting 2.5-3.0kg were housed individually and maintained under controlled conditions according to the guidelines for the use and care of animals. Diabetes was induced in rabbits by a single dose of alloxan monohydrate (100mg i.v., Sigma) and the blood glucose level was determined after one week. The animals were allocated into four groups according to the glucose levels and treatment: I (n=10) and II (n=8) had glucose levels ≥250mg/dL whereas III (n=12) and IV (n=12) had glucose level lower than 250mg/dL. The animal in the groups II and IV were treated with an ACEI, quinapril (Accupril® Pfizer) (30mg/d) added to 100g of chow. All animals were fed a 0.5% cholesterol-rich diet for 12weeks and at the end of the experiment, the glucose levels were checked again to confirm formation of groups. Daily consumption was individually and carefully monitored throughout the study. After 12weeks the animals were sacrificed with an overdose of xylazine (Rumpun, Bayer) and ketamine (Ketalar, Parke-Davis). Blood samples were obtained to biochemical analysis and liver fragments were collected for histopathological examinations.
Biochemical parameters
Blood samples were obtained after a 12-hour fast and serum glucose and total cholesterol were determined at baseline and after 12 weeks, by standard techniques using an enzymatic assay (Advia 1650, Bayer, Tokyo, Japan). ACE activity in the serum was measured by a spectrofluorometric assay, using a carbobenzoxy-phenylalanine-histidine-leucine (ZPhe-His-Leu, Sigma-Aldrich, Palo Alto, CA USA) as a synthetic substrate of ACE.31
Histopathological analysis
Specimens of liver were fixed in 10% buffered formalin (Merck), embedded in paraffin and processed for histologic and histomorphometric analysis. Sections of 4µm thickness were stained with Hematoxylin and Eosin (Merck, Germany) and Picrosirius red (F3BA, Park Davis, USA). In the sections stained with hematoxylin and eosin, histopathological aspects were determined and the fibrosis was analyzed by Metavir classification,32 in five-point scale (F0- F4), whereby F0 indicates fibrosis is absent, F1 is portal/perivenular fibrosis without septa, F2 is fibrosis with a few septa, F3 is numerous septa but cirrhosis is absent, and F4 indicates cirrhosis present. Steatosis degree was assessed, with S0 indicating ≤ 2% affected hepatocytes, S1 as 3-29% affected, S2 with 30-59% affected, and S3 with ≥60% affected hepatocytes.33 The sections stained with Picrosirius were submitted to polarized light to quantify the collagen in digitalized images of liver polarized collagen. The images with 200 dpi resolution were captured using a digital camera Olympus Q-Color3 coupled to the optical microscope Olympus BX-40, under 100x magnification. Five fields of the liver parenchyma were randomly assigned of each sample. The lens and the light intensity of the microscope were fixed, and the parameters of intensity, brightness and contrast of the image capture program were standardized for uniformity of characteristics of scanned images. All the images were captured in the same day. Colour images were first converted to greyscale images and the interval of grey shades, corresponding to the reaction was defined and applied to all images, and the area occupied by the reaction was determined by Image Tool software, version 3.0 (UTHSCSA - The University of Texas Health Science Center in San Antonio, Texas, USA).
Statistical methods
Data are expressed as the means ± SEM. Statistical analysis was performed using the nonparametric Kruskal-Wallis test followed by the post-hoc Dunn’s test. Categorical data were compared using the c2 test. Statistical significance was accepted with a p value less than 0.05. All tests were performed using the GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA).
In the parameters determined in the blood, it was observed that the four groups were hypercholesterolemic, without differences in cholesterol levels among them. ACE activity was lower in the plasma of animals treated with ACE inhibitor, groups II and IV (Table 1).
|
Groups |
n |
Glucose |
Total cholesterol |
Triglycerides |
ACE activity |
|
I |
10 |
432±45* |
1688±205 |
509±173 |
4.06±0.38 |
|
II |
8 |
514±40* |
1831±110 |
477±239 |
2.43±0.23** |
|
III |
12 |
142±9 |
1774±132 |
200±30 |
3.69±0.27 |
|
IV |
12 |
156±10 |
1571±154 |
136±17 |
2.36±0.33** |
Table 1 Plasma concentrations of glucose, total cholesterol, triglycerides and Angiotensin-Converting Enzyme (ACE) activity after 12 weeks of treatment
Values are mean±SE
*p<0.001- G I and GII > GIII and GIV; **p<0.05 - groups II and IV < I and III
I- rabbits fed a hypercholesterolemic and hyperglycemic diet; II- rabbits fed a hypercholesterolemic and hyperglycemic diet + ACEI 30mg/day; III- rabbits fed a hypercholesterolemic diet; IV- rabbits fed a hypercholesterolemic diet + ACEI 30mg/day
We verified in this model of hypercholesterolemic and diabetes type 1 rabbits, steatosis, fibrosis and inflammatory cells in the liver. The ballooning of hepatocytes (Figure 1A) and steatosis (Figure 1B) were observed near the central lobular vein. Mallory's corpuscles were not observed. Mononuclear inflammatory cells were observed along the septa following the established fibrosis (Figure 1C).
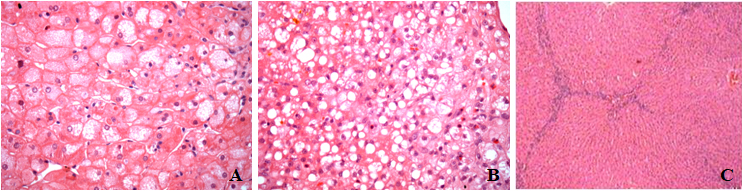
Figure 1 Photomicrograph of rabbit liver after 3months of dieting showing (A) ballooning hepatocytes; (B) – steatosis - Hematoxylin and Eosin - 400x and (C) interlobular septa - Hematoxylin and Eosin - 100x.
The liver was stained by picrosirius and analyzed with polarized light microscopy, verifying the formation of complete and incomplete interlobular septa (Figure 2). Changes histopathological findings were presented due to hypercholesterolemia, independent of the presence or absence of hyperglycemia. There was no significant difference regarding of the steatosis, between groups (Table 2). By histomorphometry, performed sections stained with picro-sirius polarized light the area with collagen, lesser degree of fibrosis found in the groups treated with ACEI compared with untreated groups, independent of blood glucose of these animals, although statistically significant difference was observed only in the group with glycemic control (group IV) (Figure 3).
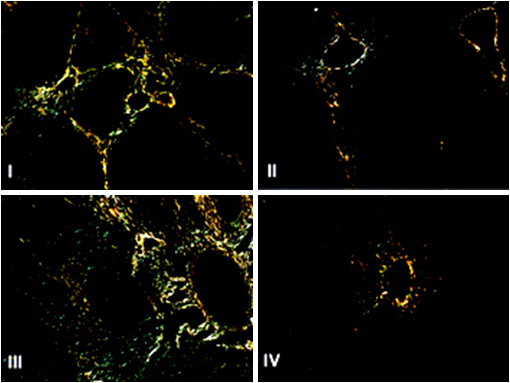
Figure 2 Photomicrograph of rabbit liver in polarized light. Picro-sirius staining (200X).
I- rabbits fed a hypercholesterolemic and hyperglycemic diet; II- rabbits fed a hypercholesterolemic and hyperglycemic diet + Angiotensin Convertin Enzyme Inhibitors (ACEI) 30mg/day; III- rabbits fed a hypercholesterolemic diet; IV- rabbits fed a hypercholesterolemic diet + ACEI 30mg/day. Groups II and IV, treated with ACEI have less collagen fibers in the hepatic parenchyma, with statistical differences only in the group that had controlled the glucose levels. (p <0.05; IV <III).

Figure 3 Fibrosis in liver of rabbits hypercholesterolemic and hyperglycemic treated with Angiotensin Convertin Enzyme Inhibitors (ACEI), after 12 weeks of diet. Collagen area determined in the digitalized image of histological slices of polarized picrosirius stained liver.
I- rabbits fed a hypercholesterolemic and hyperglycemic diet; II- rabbits fed a hypercholesterolemic and hyperglycemic diet + ACEI 30mg/day; III- rabbits fed a hypercholesterolemic diet; IV- rabbits fed a hypercholesterolemic diet + ACEI 30mg/day.
*p<0.05- IV< III; Kruskall Wallis – Dunn’s test.
|
Groups |
Fibrosis |
Steatosis |
||
|
|
F2-F4 (%)a |
Collagen (µm2)b |
S0-S1 (%)a |
S2-S3 (%)a |
|
I |
60.0 |
49772 ±18581 |
70.0 |
30.0 |
|
II |
37.5 |
16755±6705 |
87.5 |
12.5 |
|
III |
72.7 |
37529±37529 |
81.8 |
18.2 |
|
IV |
45.4 |
11572±2192* |
81.8 |
18.2 |
Table 2 Fibrosis and steatosis in liver of rabbits hypercholesterolemic and hyperglycemic treated with Angiotensin Convertin Enzyme Inhibitors (ACEI) after 12weeks of diet
aPercentage of animals showing the pathology
bCollagen area determined in the digitalized image of histological slices of polarized picrosirius stained liver *p<0.05- IV< III; Kruskall Wallis – Dunn’s test
c p=0.64; c2 test
I- rabbits fed a hypercholesterolemic and hyperglycemic diet; II- rabbits fed a hypercholesterolemic and hyperglycemic diet + ACEI 30mg/day; III- rabbits fed a hypercholesterolemic diet; IV- rabbits fed a hypercholesterolemic diet + ACEI 30mg/day
We studied the histopathological changes in the liver, since rabbits fed high-cholesterol diet accumulate lipids in the parenchyma liver inflammation and fibrosis and may have varying degrees,2 reaching even have cirrhosis. In our model, hypercholesterolemia developed triggered the development of fatty liver, inflammatory foci forming septa interlobular fibrosis. The effects of the blockade of the RAS by use of ACEI reduced the installation of liver fibrosis without fatty change, and this effect is more pronounced in the group with blood glucose control (group IV).
This protective effect in the groups receiving treatment and had lower fibrosis is consistent with the literature, which were described attenuation liver abnormalities such as fibrosis of varying degrees in experimental models34 well as antioxidant effects and anti-proliferative ACEI in the liver of animals undergoing liver transplantation.35 Therefore, treatment with inhibitor ACE can also provide a protective effect in the liver, in addition to its antihypertensive action.
The RAS is often activated in patients with liver disease chronic, such as cirrhosis,14,36 and several studies have reported that the use of blockers RAS has significant inhibitory effect on experimental development of liver fibrosis.24,30,34 HSC play an important role in fibrogenesis in the liver, there is plenty of evidence that Ang II promotes activation and differentiation of these cells into myofibroblasts. In addition, Ang II stimulates contraction and proliferation of myofibroblasts and promotes the release of cytokines inflammatory, and extracellular matrix deposition.36 Angiotensin II induces contraction and proliferation of liver cells stars, which act in the development of liver fibrosis and increases the expression of TGF-beta.37 The association of ACEI with interferon has demonstrated important attenuation in liver fibrosis in both models in vivo and in vitro, inhibiting the proliferation of HSC and messenger RNA expression procollagen I.38 In addition to the components “classics” of RAS, such as renin, ACE, Ang II and supreexpressos be the AT1 receptor in liver diseases should be considered also components of the RAS “alternative” as ACE2, Ang (1-7) and MAS receptor.39 Thus, manipulation of the RAS by blocking the SARS "classical" or stimulating SARS “alternative”, represents a potential target for antifibrotic therapy.40 Studies suggest that RAS inhibition may become a strategy antifibrotic also important to apply in clinical practice and that the modulation of the RAS ACEI and AT1 receptor blockers may be options promising therapies for the treatment of liver fibrosis.10
The results suggest a benefit in liver protection achieved by the use of an ACEI in hypercholesterolemic and diabetic rabbits, mainly if the glucoses levels were controlled. This study highlights the importance of the RAS in protecting organs affected by high levels of lipids and glucose.
Daniel Pomaro was supported by CNPq, a Brazilian foundation.
No conflict of interest.
None.

©2021 Pomaro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.