Journal of
eISSN: 2373-4396


Review Article Volume 9 Issue 2
Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), India
Correspondence: Vasudevan Dinakaran, Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry-605006, India, Fax 1
Received: January 17, 2017 | Published: June 28, 2017
Citation: Dinakaran V (2017) Clinical Implications of Circulating Microbiome in Cardiovascular Disease Patients. J Cardiol Curr Res 9(2): 00317 DOI: 10.15406/jccr.2017.09.00317
The circulating microbiome or the blood microbiome has been analysed recently in chronic inflammatory diseases like obesity, diabetes, cirrhosis and cardiovascular diseases (CVDs) in humans. Evidences that microbial components in blood could play a crucial role in the pathophysiological events leading to or during the course of disease continue to mount. The blood microbiome has been investigated primarily by profiling the circulating bacterial and viral elements using high-throughput sequencing technologies. However, the clinical implications of circulating microbiome in humans remain largely unexplored. The evidence for the presence of a stable blood microbiome in CVDs is still questionable. Nevertheless, there is evidence for a link between the circulating microbiome in obesity, diabetes and CVDs, indicating the influence of physiological, biochemical and environmental factors on the circulating microbiome in metabolic diseases. With this background, we explore the reports on the clinical consequences of bacteremia in CVD patients which formed the basis for the analyses on the topic “circulating microbiome”.
Keywords:circulating microbiome, clinical manifestations, tissue microbiota, systemic diseases, metabolic syndrome
Over the past two decades, the hypothesis that metabolic diseases have their origin in chronic low grade inflammation has been validated in several studies.1 However, the antigens responsible for the onset of metabolic inflammation have not been researched extensively. Blood which was normally believed a sterile milieu has been found to contain microbial load in CVDs in recent years.2 The circulating DNA in CVDs has been widely analyzed,3,4 per contra, there are very few reports on the presence of circulating microbial DNA in CVDs. Circulating microbiome encompasses the entire microbial habitat of circulation, including, microbes, their genomes and genes and the circulatory environment. The term blood microbiota includes microorganisms and their products in blood. The question of existence of a circulating microbiome in systemic diseases was propounded based on the evidence for the presence of microbes in the circulation of systemic disease patients. Analyses on the circulating microbiome were initiated after profiling the skin, oral, respiratory, gut and urogenitary microbiome by large-scale sequencing. The blood microbiome could be a source of antigens responsible for the onset of metabolic diseases such as, obesity, diabetes and CVDs.
The first report on the presence of circulating microbiome was a large cohort longitudinal study involving obese and diabetic patients.2 Remarkably, a similar blood microbiome was present in diabetic population and the population destined to develop diabetes. Subsequently, blood microbiome was profiled in end-stage renal disease ESRD patients.5 Gut bacteria were identified in the circulation of ESRD patients and these patients had higher levels of inflammatory markers like high sensitive C-reactive protein (CRP), interleukin-6 and lactate in plasma, indicating that Gut bacteria translocation is associated with micro-inflammation in ESRD patients. In continuation with this, presence of blood microbiota and its long-term prognosis in CVD patients was studied for the first time which concluded that perturbations in blood microbiota equilibrium is associated with or linked with the onset of the disease.6 Successively, another paper on the presence of circulating microbiome in CVD patients presented similar findings.7 Comprehensively, these publications concluded that Proteobacteria were the dominant circulating bacterial population in CVDs. These findings led to the hypothesis that the presence of blood microbiome could be one of the initial steps leading to metabolic diseases like obesity, diabetes and CVDs. With these preliminary informations, the presence of a stable blood microbiome in these diseases could be evidenced. The involvement of inflammation in the development of these diseases and the pro-inflammatory effects of circulating microbial components could reflect the influence of these microbes in the clinical complications of the disease.
Indeed, recent research has proved and analyzed the influential role of gut microbiota on innate immunity and its profound impact on CVD complications. Consistently; the effect of oral microbiota in the pathogenesis of these diseases has been largely explored. The blood microbiota studies form the first line evidence for the involvement of tissue microbiota in these diseases and also provide some insights into the mechanism of action of circulating microbiota. Obviously, these studies were observational or qualitative in nature and could not demonstrate the causative or influential role of blood microbiota in the onset of these diseases.
Moreover, these studies were preliminary and the results obtained need to be replicated in further research involving larger number of samples to authenticate the presence of a stable blood microbiome in these diseases. Besides, the bacterial DNA could represent an innocent bystander in the disease process. The mechanisms regulating blood microbiome symbiosis and the molecular crosstalk between the host and microbiota have to be further delineated. Pathological studies of human lesions have identified a remarkable correlation between intra-plaque hemorrhage, extensive necrotic core size with plaque rupture and clinical events.8‒10 Hence, in the present article, the reports on the clinical inferences of bacteremia in CVD patients were discussed and analysed from a microbiome perspective.
Clinical implications of circulating microbiome in CVDs
The presence of circulating microbiome in cardiac patients was identified for the first time by Amar et al.,6 An increased Proteobacteria population in CVDs correlated with major factors like age, blood pressure, fibrinogen and alanine aminotransferase (ALT) levels. Moreover, a 3.7 fold increase in the risk of incident acute cardiovascular events, such as, myocardial infarction (MI), patients under revascularization therapy, significant myocardial ischemia and cranial infarctions were found in volunteers with the highest levels of Proteobacteria than those with the lowest levels of Proteobacteria. This is the only study on the association of blood microbiota with CVDs as on date.
Subsequently, Rajendhran et al.,7 described a dysbiosis in the circulating microbiome in CVD patients as compared to clinically healthy subjects by amplicon sequencing of the 16S rRNA gene and observed similar results as the aforementioned study. Successively, the same group analyzed the circulating microbiome and virome by shotgun sequencing of plasma in CVD patients. Nevertheless, the number of samples analyzed in this study was far too less. The authors also demonstrated a significant correlation between circulating bacterial elements and host factors like blood pressure, heart rate, C-reactive protein (CRP) and Procalcitonin (PCT).11 Recently, Sato et al.,12 detected viable gut bacteria in the circulation of diabetic patients. In CVDs, bacteremia leads to the formation of plaque microbiota (atheroma) in atherosclerosis, endothelial valve lesions in Rheumatic heart disease (RHD), coronary endothelial damage in coronary artery disease (CAD), vascular dysfunction in arteriosclerosis and atherosclerosis and endocardial damage in infective endocarditis (IE). The implications of bacteremia in CVDs have been briefly outlined in Figure 1 and tabulated in Table 1.
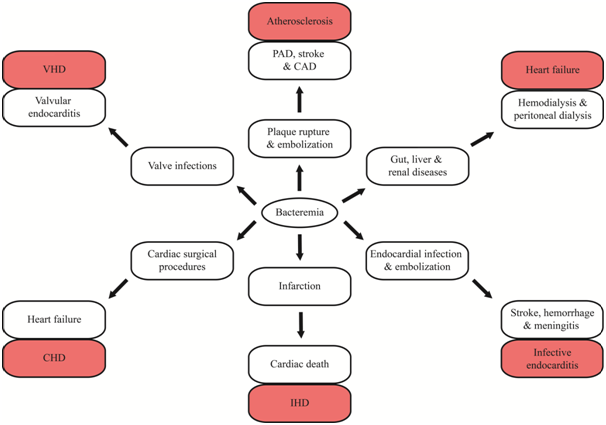
Figure 1 Schematic representation of clinical implications of bacteremia in cardiac disease patients. VHD; Valvular Heart Disease; CHD: Congenital Heart Disease; IHD: Ischemic Heart Disease; PAD: Peripheral Artery Disease; CAD: Coronary Artery Disease.
|
S. No. |
Cardiovascular Disease (CVD) |
Initiating Event |
Resulting Complications [Reference] |
|
1 |
Atherosclerosis |
Bacteremia |
Peripheral artery disease, cerebral and |
|
ischemic events16 |
|||
|
Plaque rupture |
Acute coronary syndrome17 |
||
|
Embolization of plaque debris |
Cerebral dysfunction and other neurological complications21 |
||
|
Circulating endotoxemia |
Systemic immune response and circulating immune complexes22 |
||
|
2 |
Valvular heart disease (VHD) |
Bacteremia due to intravenous drug abuse |
|
|
Systemic immune response |
Circulating immune complexes28 |
||
|
3 |
Myocardial infarction (MI) |
Bacteremia and viremia |
Elevated C-reactive protein (CRP) levels and increased incidence of cardiac mortality31 |
|
and Coronary artery disease (CAD) |
Circulating endotoxemia |
Systemic immune response and circulating immune complexes38, 39 |
|
|
Circulating HSP70 |
Systemic inflammatory response in MI108 |
||
|
4 |
Infective endocarditis (IE) |
Circulating immune complexes |
Longer duration of illness, extra-valvular manifestations, glomerulonephritis, polyarteritis and Osler nodes35, 41 |
|
Anticardiolipin IgG antibodies |
Q fever endocarditis36 |
||
|
Embolization |
Ischemic stroke, subarachnoid hemorrhage, meningitis, brain abscesses and mycotic aneurysms51 |
||
|
Hemodialysis and peritoneal dialysis |
Nosocomial IE54‒ 57 |
||
|
Large vegetation |
Congestive heart failure and stroke59 |
||
|
Mitral and aortic valve vegetation |
Abscess formation60 |
||
|
Bi-prosthetic valve infections |
Valve ring abscess and cuspal infections62 |
||
|
Acute bacterial endocarditis |
|||
|
Xenograft or mechanical valves |
Recurrent infections79 |
||
|
Circulating endotoxemia |
|||
|
5 |
Heart failure |
Surgical procedures like CABG and PCI |
|
|
Bacteremia and viremia |
Cardioembolism and arterioarterial embolism82 |
||
|
6 |
Other Ischemic and Congenital heart diseases |
Chronic bronchitis |
Cerebrovascular ischemia83 |
|
Circulating immune complexes |
Heart disease84 |
Table 1 Clinical implications of bacteremia in Cardiac disease patients
Atherosclerosis
Atherosclerosis is a condition resulting from the accumulation or deposition of plaques on the inner walls of coronary arteries. Circulating bacteria and their components can foster plaque formation and aggravate the pathology of the disease. Polybacterial infection fits well with the concept of “infectious burden” in atherosclerosis.13,14 Stable plaques in coronary arteries are usually clinically silent or may eventually lead to stable Angina pectoris. Vulnerable atherosclerotic plaques are more susceptible to destabilization and rupture, compared to stable fibrotic plaques, leading to clinical events characterized by increased infiltration of leukocytes and other inflammatory mediators.15,16 Bacteremia may contribute to vascular thrombosis in Peripheral artery disease, which can predict cardiac and cerebral ischemic events.17 Atherosclerotic plaque rupture is the initiating event resulting in most acute coronary syndromes including unstable angina and MI.18
Drug eluting stents which were earlier a source of external infection have been used to overcome the problem of restenosis and thrombosis, as they inhibit vascular smooth cell proliferation and regrowth of the endothelium.19 Of late, bio-absorbable stents were developed and used widely after successful completion of the first clinical studies.20 Penetration of atherosclerotic plaques in the aortic vasa vasora may result in intra-mural hematoma of aorta and ischemia ending in organ dysfunction.21 Atherosclerotic debris embolize to other organs, like brain leading to cerebral dysfunction and other neurological complications.22 Circulating endotoxemia and induced systemic inflammatory response in atherosclerosis patients stimulate host immune cells to release pro-inflammatory particles like cytokines and chemokines in circulation.23 Oral microflora has been reportedly implicated in bacteremia in atherosclerosis patients.24 Viral infections have been noted in more number of atherosclerotic cases than other CVDs, with most of them being identified in plaques.25
Valvular heart disease (VHD)
Valvular heart diseases (VHD) involve damage or defects in the cardiac valves. Analysis of the circulating microbiome has revealed that bacteria were more enriched in VHD patients than congenital and ischemic heart disease (CHD and IHD) patients with a preponderance of Proteobacteria population.7 Rheumatic heart disease (RHD), resulting from rheumatic fever caused mostly by Group A Streptococcal infection, is the only cardiac disease with a well-established infectious origin. Elevated circulating microbiome concentration in VHDs may contribute to increased infectious burden; aggravate the endothelial valve lesions by polymicrobial infection of the inflamed valves, with the infection subsequently spreading to the inner endocardial wall in IE.
Bacteremic events following rheumatic fever are primarily responsible for valvular infections in RHD. Complete dental assessment is considered essential prior to any valvular surgical procedure in developed nations. Tricuspid valve endocarditis results chiefly because of intravenous drug abuse, long term central venous catheter (CVC) infections, and implanted cardiac device infections and to a lesser extent, from impaired host defense. Tricuspid pathological lesions, characterized by stenosis and regurgitation are caused due to rheumatic, carcinoid and collagen vascular diseases. Tricuspid valve infections were seen in more than 50 % of intravenous drug abuse patients with IE.26,27 Valvular stenosis resulting primarily from rheumatic fever induces regurgitation and causes valvular atresia. Valvular septal defects after infection may result in valvular IE. Elevated levels of circulating immune complexes like acute phase proteins, cytokines and chemokines have been oftentimes observed in VHD cases.28,29
Aortic valve disease, characterized by aortic stenosis and aortic insufficiency is caused due to calcific degeneration of the congenital mitral valve and RHD.30 Mitral stenosis and the accompanying valve lesions, which are predominantly regurgitant in nature, are the most common causes of aortic valve disease. Mitral valve infections in RHD further intensifies the valve lesions. Approximately, 60 % of patients presenting with mitral stenosis have a previous incidence of rheumatic fever.31 Mitral insufficiency results because of floppy mitral valve, with ischemia and endocarditis. Oral hygiene and dental bacteria have been implicated in bacteremia in VHD.32,33
Myocardial Infarction (MI) and Coronary artery disease (CAD)
CAD results due to accumulation of plaques or fatty acids in coronary arteries of the myocardium. Thrombus or clot formation in coronary vessels which may not be infectious obstructs blood flow to the myocardial tissue ending in infarction. Rupprecht et al.,34 showed that bacterial and viral load in circulation were correlated positively with CRP levels and future cardiac mortality in CAD, indicating that infectious burden concentration aids in the long term prognosis of CAD patients. Surgical coronary revascularization procedures, like Percutaneous coronary intervention (PCI) and Coronary artery bypass Graft (CABG) have been known to induce circulating endotoxemia in CVD patients.35‒37 Circulating endotoxemia induced elevated immune response has been shown in CAD patients.38,39 Infection of the arterial wall results in localized dilatation, progresses to abscess formation and embolization of vegetation at vessel branch points, leading to the formation of mycotic aneurysms. Embolization of vegetation to the central nervous system results in neural ischemia.
Infective endocarditis (IE)
Infective endocarditis (IE) is usually a bacterial infection of the endocardium or equivalent prosthetic heart surfaces and is an important cause of cardiac infections. IE is the result of bacteremia, endothelial injury, altered hemodynamics and injury to the valves. Circulating microbiome in IE could elevate systemic immune response resulting in some of the complications, such as, hypergammaglobulinemia, splenomegaly, and presence of macrophages in blood and production of rheumatoid factors. Deposition of immune complexes and complement factors in the infected lesions contribute to systemic manifestations of IE, such as, longer duration of illness, extra-valvular manifestations, glomerulonephritis, polyarteritis and Osler nodes.40,41 Increased levels of anticardiolipin IgG antibodies in acute Q fever were strongly correlated with the progression to Q fever endocarditis.42 Kauffmann et al.,43 described higher levels of circulating immune complexes in patients with signs of renal involvement or cutaneous vasculitis than in patients without these extra cardiac manifestations. In addition, elevated levels of circulating immune complexes such as, acute phase proteins, cytokines and chemokines have been detected in nearly all IE cases.41,43
Most common etiological agents of bacteremia in IE include S. aureus,44,45 coagulase negative Staphylococc. 46 group A, group B47 and group D Streptococci48 and HACEK microorganisms.49 Some of the rarely identified organisms include P. aeruginosa,50 Lactobacillus,51 anaerobic bacteria,52 Bacteroides53 and other Gram negative bacteria.54 Coagulase negative Staphylococci were the most common cause of early Prosthetic valve endocarditis (PVE).46,55 Transient bacteremia has been frequently reported in endocarditis.56
Embolization is another serious complication of IE and neurological embolization results in Stroke. Microbes in circulation could promote embolization. In high risk, critically ill patients with left-sided IE, complications like ischemic stroke, subarachnoid hemorrhage, meningitis, brain abscesses and mycotic aneurysms were seen.57 In other studies, larger vegetation with significantly higher levels of serum C-reactive protein (CRP) and lower albumin concentrations were found in younger patients with incidence of embolism had than those without embolism. Intriguingly, no association was observed between embolism and gender, site of infection or involved microorganisms.58 Embolic or metastatic events were more commonplace in IE, but were not associated with significant mortality.59
ESRD patients receiving chronic hemodialysis were particularly prone to non-nosocomial IE60‒62 than those receiving peritoneal dialysis.63 S. aureus and MRSA blood stream infections were more prevalent in hemodialysis patients.64 Clinical relevance of bacterial vegetation, visualized by echocardiography in IE has been reported in many earlier studies. Large vegetation in IE cases was associated with higher number of surgeries and not with increased incidence of stroke or death, more specifically; aortic valve vegetation was associated with a higher incidence of congestive heart failure and stroke.65 Mitral valve vegetation had a significantly higher incidence of embolic events than aortic valve vegetation, while, the incidence of abscess formation was higher in aortic than in mitral valve endocarditis.66
Intravenous catheter (IVC) infections might result in an increased circulating infectious burden in a very short period ending in MI or exacerbating the earlier existing CAD. CVDs caused by IVC infections might result in increased endocardial damage and the infectious burden may spread rapidly resulting in early endocarditis.67 Frequency of valve ring abscesses and cuspal infections were higher in IE involving biprosthetic valves.68 Valve ring abscesses of the heart were a frequent complication of acute bacterial endocarditis.69,70
Cardiac device endocarditis includes infection of the prosthetic valves and implantable devices. PVE is caused by external perioperative infection either during surgery or in the intensive care units. Prosthetic valve infection causes tissue damage facilitating the accumulation of platelets, fibrinogen, fibronectin and plasma proteins at the valve prostheses, sutures, and mechanical and inflammatory lesion sites. Staphylococci, particularly, S. aureus and S. epidermidis, owing to their biofilm forming ability, were the most identified species in prosthetic valves71,72 while Enterococci were seen in late forms of PVE. The bacterial growth leads to separation between valve and tissue resulting in paravalvular leakage.72,73 Peripheral venous catheter (PVC) infections74 and central venous catheter (CVC) infections75 mostly result in IE.
Other than S. aureus, P. aeruginosa and some fungal species were responsible for severe forms of IE.76,77 Involvement of multiple valves, native valves, and large vegetation were mostly seen in endocarditis due to Streptococcus bovis. A significantly higher rate of occurrence of embolism was noted in S. bovis group.78 Selton-Suty et al.,79 observed a lower rate of emboli formation in elderly people with IE and the predominant bacterial species were S. aureus, Enterococci and group D Streptococci. In another study, the baseline characteristics of IE patients and the nature of injured valves correlated with specific microorganisms detected in blood.80 Bacteremia, identified by persistent positive blood cultures doubles the risk of left sided IE.81
Nosocomial IE, most often a consequence of surgical procedures or indwelling catheters was frequently caused due to MRSA infection and leads to bacteremia as evidenced by positive blood cultures. In a survey, 30 % of IE was associated with health-care settings with MRSA or Enterococcus infection, than factors like age, hyperglycemia, renal impairment and heart failure.67 Approximately, 10 % of nosocomial bacteremia cases reportedly result in IE,82 accounts for 20 % of IE cases83 and has a higher mortality rate in critically ill patients.83,84 Homograft valves and pulmonary autografts are less prone to recurrent bacterial endocarditis than xenograft or mechanical valves.85 Chronic and recurrent infections in IE were chiefly caused by Staphylococcus by switching their phenotype into small colony variants.86
Other Ischemic and Congenital heart diseases (IHD and CHD)
Blood stream infections and their cardiac manifestations have been noted in IHD also. Pasceri et al.,87 detected H. pylori strains in the circulation of more number of IHD patients (62 %) and the bacterial strains were associated with factors like age, sex and social class. Both bacterial and viral infections in cerebrovascular ischemia patients were shown to elevate the risk of cardioembolism and tend to increase the risk of arterioarterial embolism.88 Also, frequent or chronic bronchitis was associated with cerebrovascular ischemia.89 Furthermore, elevated levels of circulating inflammatory cytokines like tumor necrosis factor α (TNFα), interleukin-6, interleukin-10, and soluble CD14 (sCD14) were correlated with bacterial endotoxin levels in CHD patients.90 Surgery for congenital heart defects like valvular aortic stenosis,91 aortic stenosis (AS), pulmonary stenosis (PS), or ventricular septal defect (VSD)92,93 result in IE.
Heart failure
Microbial components in circulation of heart failure cases were mostly due to the translocation of microbes from gut in gastrointestinal and renal injuries. Ischemic injury with increased gut permeability owing to disrupted gut mucosal structure and function can lead to microbial translocation.94 Circulating endotoxemia has been largely identified in chronic and congestive heart failure patients presenting along with other diseases.95,96 Additionally, surgical procedures like CABG and PCI can induce systemic endotoxemia which, on long term can result in heart failure.32,34 In a pilot study involving patients undergoing CPB, Oudemans-van Straaten et al.,97 proposed a relation between post-perfusion syndrome and endotoxin translocation from the gut. LPS (endotoxins) has been widely studied as an important source of inflammatory stimuli in atherosclerosis.23,36
Circulating LPS has result in atherosclerosis in peritoneal dialysis patients.98 Subclinical endotoxemia has been reported to result in a three-fold increased risk of incident atherosclerosis and CVD.22,99 Circulating endotoxins also influence cardiac troponin T levels.100 Circulating LPS and sCD14 were associated with hypertension in HIV-infected individuals.101 Furthermore, HIV replication and antiretroviral therapy with elevated LPS levels were associated with atherosclerosis.102
LPS in blood, with a variety of proatherogenic properties induces dilatation of resistance arteries leading to reduced arterial pressure and decreased renal perfusion103,104 and were normally accompanied by increased levels of circulating inflammatory molecules, such as CRP, cytokines and chemokines. Pro-inflammatory cytokines can induce inflammation in endothelial vessels, as well as, proliferation of local monocytes or macrophages and vascular cells.105‒107
Thus, from the initial studies, it is evident that microbial components exist in the circulation of CVDs which are normally recognized as non-infectious and non-communicable in nature and these components might have potential implications in the clinical sequelae of CVDs. Nonetheless, microbes in circulation cannot be confirmed as etiological agents in CVDs except RHDs. Most cardiovascular deaths were a result of resultant IE implying that, these microbes would have been acquired during the progression of the disease. Consequently, the circulating microbiome load and diversity is dependent on the clinical condition and immune status of the disease. Blood microbiota in CVDs is dependent on patient characteristics like age, gender, glycemic status (diabetes mellitus), obesity, hypertension, smoking or tobacco usage, alcohol consumption and genetic predisposition. Besides these factors, from the extensive research during the last decades, it is evident that diseases of the gut and oral cavity have a strong impact on the blood microbiome in CVDs. Circulating microbiome can also be introduced from external source by the usage of prosthetic devices, implantable cardiac devices, and by intravenous drug abuse and this can provoke the complications of CVDs. The development of the disease could determine the microbiome diversity and load, thus elevating the infectious burden which in turn, could exasperate the disease.
Vasudevan Dinakaran acknowledges the funding through DST-SERB Project Assistant Fellowship.
None.
The author declares that there are no conflicts of interest regarding the publication of this manuscript.

©2017 Dinakaran. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.