Journal of
eISSN: 2373-4396


Case Report Volume 13 Issue 1
Department of Echocardiography, CPC - Private De Cardiology Center, Argentina
Correspondence: Juan I Cotella, Department of Echocardiography, CPC - Private De Cardiology Center, Argentina, Tel 5493874869170, Fax 543814311009
Received: January 24, 2020 | Published: February 18, 2020
Citation: Cotella JI, Prado DA. Cardiac amyloidosis the contribution of images for diagnosis. J Cardiol Curr Res. 2020;13(1):21-23. DOI: 10.15406/jccr.2020.13.00466
The restrictive pathologies are a group of diseases of low prevalence. Cardiac amyloidosis is considered to be the prototype of the infiltrative form of restrictive cardiomyopathies. The myocardial involvement is frequent, even at the first appearance of the disease and it has a poor prognosis; and although pleural involvement is less frequent, its presence determines a much more torpid evolution than isolated cardiac presentations. The main problem that these pathologies concern is their late recognition and the consequences this implies in the patient evolution. Nowadays, cardiac images tools may facilitate the diagnosis, allowing an early detection. We share an unusual case of amyloidosis with pleural involvement, where the results of the echocardiogram and the cardiac magnetic resonance allowed an approach to an accurate diagnostic.
Keywords: amyloidosis, restrictive, infiltrative, magnetic resonance, echocardiogram
IR, inversion recovery; LV, left ventricle; CPR, cardiopulmonary resuscitation; MRI, magnetic resonance imaging
The group of diseases associated to abnormal diastolic function with restrictive pattern is very rare in clinical practice.1 Amyloidosis represents one of the most characteristic prototypes of these pathologies. Ambiguity in clinical practice leads to a late and inaccurate diagnosis, performed correctly in less than 20% of patients.2 Cardiac image studies are important for the early diagnosis; the echocardiogram is one of the main tools in the diagnostic algorithm. The case we developed hereafter is an example of a common setting of the appearance of this pathology, revealing that if there is no clinical suspicion, the probabilities to achieve an accurate diagnosis are low.
The case is about a female patient of 65 years old, obese, with dyslipidemia, hypertension, asthma, with a background of dyspnea NYHA class III of 3 months of evolution and a weight loss of 10kg. During this period, she referred many medical visits due to these symptoms. In one of them, an electrocardiogram was performed, with a pattern of QS from V1 to V6 observed on it. This motivated to perform an echocardiogram, reporting concentric hypertrophy and mild pericardial effusion. Then, a myocardial perfusion imaging was performed, showing a severe anterior and lateral ischemia. Finally, a coronary angiography was performed, exhibiting absence of coronary stenosis.
Subsequently, she was admitted to our institution with a clinic diagnosis of acute heart failure, Stevenson B. A chest X-ray showed bilateral pleural effusion, grade II. During the admission, the only significant finding was mild anemia (Hb 11,1g/dl). A transthoracic echocardiogram (Figure 1 & Video 1) was done showing normal left ventricle diameters, LV ejection fraction 47%, mild concentric hypertrophy, biatrial enlargement, mild to moderate mitral regurgitation, pseudo normal ventricular filling pattern, e 4cm/sec, E/e´ ratio greater than 18, moderate tricuspid insufficiency, an estimate pulmonary artery systolic pressure of 55mmHg and mild pericardial effusion. The presumptive diagnosis of infiltrative restrictive cardiomyopathy was assumed. A myocardial 3D strain imaging with speckle tracking was done, showing normal longitudinal strain of apical segments and a strong impaired values of basal and mid segments (-16 to -28 at apical segments vs 4 to -4 at basal level) (Figure 2C). The patient improved clinically with diuretics and vasodilators according to local guidelines. Nevertheless, during the third day of hospitalization she experiences a sudden death episode, with ventricular tachycardia documented in the telemetric system, with an adequate response to cardiopulmonary resuscitation (CPR).
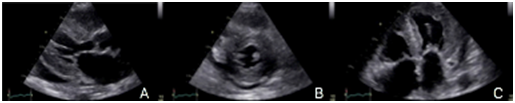
Figure 1 We can see a parasternal long axis view (A), short axis at mid ventricular level (B) and 4 chambers (C), showing mild hypertrophy (12mm) with biatrial enlargement and mild pericardial effusion (White arrow).
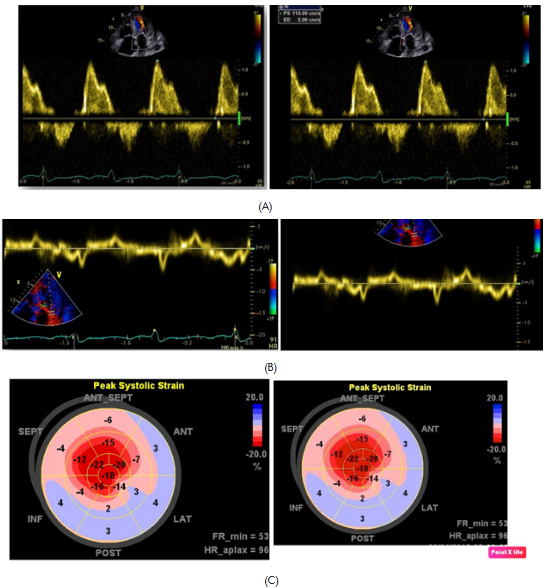
Figure 2 diastolic mitral flows with pseudo normal pattern (A). E´ wave plainly decreased to the side mitral ring level (B). Global longitudinal strain with normal values at apical level and diminished in mid and basal segments (C).
Video 1 parasternal long axis, shot and apical axis view. 2D, color doppler, showing mild hypertrophy with biatrial enlargement, mild pericardial effusion, mild to moderate mitral regurgitation, moderate tricuspid regurgitation, inferior cava vein dilated.
An MRI (Figure 3) was performed, reporting during inversion recoverysequences (IR) of late gadolinium enhancement the impossibility to diminish properly the left ventricle due to its global involvement and the hypo-intensity signal at intra-cavitary level, despite performing IR sequence 5 (five) minutes after contrast injections. During look Locker sequence, an impairment of more than 50% of the left ventricle myocardium was found. Leading the diagnostic suspicion of amyloidosis, a pleural puncture was performed revealing 6g/dl of protein in the exudate with a high number of lymphocytes, polymorph nuclear leucocytes, plenty of atypical mesothelial cells and a low number of atypical plasmatic cells. Bacterial and mycobacterial culture were negative.Then, a fine needle aspiration biopsy of abdominal fat was made, giving a positive result for amyloid.The patient was moved to the haemato-oncology unit in order to evaluate an alternative treatment and she died 22days later.
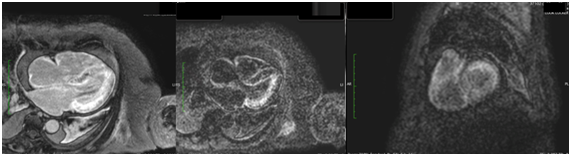
Figure 3 Sequence of late enhancement in short axis and in the four chambers. Impossibility to dim the myocardium properly due to global involvement, together with intracavitary hypo-intensity. Sub-endocardial and sub-epicardial diffuse involvement in left ventricle, as well as in atrial myocardium and intra-atrial septum.
Cardiac amyloidosis was considered an uncommon disease, however, nowadays; there is a suspicion that the low prevalence is associated to the deficiency or delay in the diagnosis.6 These mistakes are multifactorial, especially due to the appearance diversity, the early low suspicion and the lack of proper diagnostic strategies.
Amyloidosis means a group of diseases of different etiologies with a common characteristic: the amorphous material deposit at extracellular level, affecting the heart, liver and other tissues. Even though clinical and image characteristics of different types of amyloidosis have a lot in common, therapeutic strategies and prognosis are usually different in each of them.
Among the ones affecting the myocardial tissue more frequently, there are two types: those associated to light chains (AL) production, and those associated to transthyretin (TTR). Knowing the difference between them helps to an early diagnosis and, of course, an individual therapeutic approach. In the US, there is around 3,000 new cases yearly.4 The clinical appearance usually varies, being heart condition the main defining prognosis. Myocardial involvement is usually shown by symptoms as heart failure and atrial and ventricular arrhythmias.
The physio-pathological mechanism, by which the systolic and diastolic dysfunction is built, is determined by both the direct amyloid deposit and the “toxic” effect of the deposited and circulating protein parent.5 The presence of heart failure of underlying cause, associated to hepatomegaly and proteinuria should be the first diagnostic suspicious. Considering this situation, an electrophoretic study must be performed to quantify the presence of protein chains (kappa y lambda). The tissue biopsy, to confirm the amyloid deposit, is an essential element for diagnosis able to be obtained by aspiration of abdominal fatty tissue. The bone marrow puncture allows establishing the specific clonal diagnosis. The endomyocardial biopsy is only necessary in less than 15% of patients; however, the specificity is 100%.6,7
Imaging studies are clearly useful; nevertheless, they do not show specific data to differentiate the diverse types of diseases. Classic data include normal size ventricles with mild hypertrophy, biatrial enlargement, changes in the ventricular filling pattern (pseudo normal or restrictive pattern) and pericardial effusion. The myocardial assessment with longitudinal strain shows a very important characteristic pattern for the diagnosis, characterized by strain values diminished to basal level and mid segments, with normal values of apical segments. These findings may be present in absence of ventricular motility disorders, so the benefit is understandable8 Our patient presented all these features.
With regards to electrocardiographic evidences, we recalled that in our case a pseudo-infarction image in the front-side face was found. These manifestations are usually present in less than the half of patients. The presence of pleural effusion described in our patient is usually present in less than 6% of patient with myeloma, nevertheless, incidence of pleural infiltration is of less than 0,8%,10,11 being associated to a mean of livelihood of 1,5-3months.12
AL type amyloidosis diagnosis may be performed in 95% of cases where the combination of light chain findings in plasma and urine are present, through electrophoretic studies of immunofixation and fatty tissue aspiration. Even then, the previous diagnosis is important and, at this point, image methods are defining. Whereby, in light of the distinctive echocardiographic findings, including the longitudinal strain, or the changes observed in MRI studies, added to the detection of monoclonal protein expression, there is an approach to the diagnosis to a large extent, without being necessary the bone marrow biopsy puncture nor the endomyocardial biopsy.
Annual mortality of patients with amyloidosis and heart involvement reach yearly numbers of 24%, and in those cases where death is present in the first six months, half of them are sudden death.13,14 The grade of heart condition is a determining prognosis, and in this sense, MRI is a heavy weight prognosis attribute through determination of extracellular involvement grade.15
Cardiac amyloidosis is considered an uncommon disease but should be consider as a possibility in some patients admitted due to cardiac heart failure without a clear etiology. Most typical echocardiography findings include normal size ventricles with mild hypertrophy, biatrial enlargement and changes in the ventricular filling pattern. Global longitudinal strain has been shown as a very useful echo tool providing a very important characteristic pattern for the diagnosis.
None.
Author declares that there are no conflicts of interest towards publication.
None.

©2020 Cotella, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.