Journal of
eISSN: 2373-4396


Review Article Volume 3 Issue 3
Department of Medicine and Cardiovascular Research Group, University of Alberta, Canada
Correspondence: B I Jugdutt, Cardiology Division of the Department of Medicine and Cardiovascular Research Group, 2C2 Walter MacKenzie Health Sciences Centre, University of Alberta, Edmonton, Alberta, T6G 2R7, Canada, Tel (780) 439-0745, Fax (780) 437-3546
Received: April 23, 2015 | Published: August 19, 2015
Citation: Jugdutt BI (2015) Aging-related Changes in Cardiac Extracellular Matrix: Implications for Heart Failure in Older Patients. J Cardiol Curr Res 3(3): 00101. DOI: 10.15406/jccr.2015.03.00101
The aging population with heart failure (HF) is increasing worldwide. Hypertension (HTN) and myocardial infarction (MI) are the two main comorbidities leading to HF in the elderly (age ≥ 65years). Aging is progressive and results in cardiovascular changes that lead to an aging phenotype and negatively impact disease expression and response to therapy. Aging-related changes contribute to adverse cardiac remodeling and HF with preserved ejection fraction (HFpEF). HTN also leads to HFpEF whereas MI leads to HF with reduced EF (HFrEF). Aging and concomitant HTN or MI accelerates the march to HF. The cardiac extracellular matrix (ECM) is critical for maintaining cardiac shape/function. A key mechanism in the development and progression of HF due MI and HTN involves adverse cardiac ECM remodeling. Disruption of the ECM network and dysregulation of ECM homeostasis and metabolism result in adverse cardiac remodeling with shape deformation and dysfunction that lead to HF, disability and death. Aging-related cardiac remodeling with superimposed progressive left ventricular remodeling leading to HFpEF or HFrEF in older patients is a persistent problem that has important therapeutic implications. Studies suggest that in the elderly, novel pathways can be targeted for optimizing therapy in HFrEF post-MI and HFpEF post-HTN. Therapeutic strategies that include targeting of adverse cardiac ECM remodeling could prevent/limit/reverse progression to HF in aging patients.
Keywords: aging, extracellular matrix, hypertension, myocardial infarction, cardiac remodeling, heart failure
CV,cardiovascular; ACC, american college of cardiology; ACE, angiotensin-converting enzyme; ADAM, a-disintegrin and metalloproteinase; AGES, advanced glycation products; AHA, american heart association; AngII, angiotensin II; ARB, angII-Type 1 receptor blocker; CMR, cardiac magnetic resonance; CRP, C-reactive protein; ESC, european society of cardiology; CGMP, cyclic guanosine monophosphate; ECM, extra cellular matrix; ENOS, endothelial nitric oxide synthase; GAG, glycosaminoglycan; Gal, galectin; GDF-15, growth differentiation factor 15; HF, heart failure; HFREF, HF with reduced ejection fraction; HFPEF, HF with preserved ejection fraction; HTN, hypertension; IL, interleukin; IL-1Ra, IL-1 receptor antagonist; INOS, inducible nitric oxide synthase; LV, left ventricular; MCSF, macrophage colony stimulating factor; MI, myocardial infarction; MMP, matrix metallo proteinase; MPO, myeloperoxidase; MT-MMP, membrane-type MMP; MRA, mineralocorticoid receptor antagonist; NGAL, neutrophil gelatinase-associated lipocalin; NNOS, neuronal nitric oxide synthase; OPN, osteopontin; PCI, percutaneous coronary intervention; PKG, protein kinase G; RAAS, renin aldosterone angiotensin system; ROS, reactive oxygen species; TGF, transforming growth factor; SLPI, secretory leucocyte protease inhibitor; SPARC, secreted protein acidic and rich in cysteine; STEMI, ST segment elevation MI; TIMP, tissue inhibitor of MMP; TNF, tumor necrosis factor
Heart failure (HF) is a major cause of disability and death worldwide and predominates in the older population.1‒7 Since the 1950s, the HF burden in the older population has been been rising steadily in the United States of America, Europe and other developed countries.1,2,6‒8 Importantly, this rising trend has persisted despite steady progress in HF therapies that are recommended in the management guidelines and updates published by the American Heart Association (AHA), American College of Cardiology (ACC), and European society of Cardiology (ESC).1‒5,7 Left unchecked, this alarming upward trend could overtax the available resources of health care systems worldwide.9 Over the last 5 decades since the mid-1970s, four pertinent advances were made. First, population studies identified that antecedent myocardial infarction (MI) and hypertension (HTN) are the two leading causes of HF in older people who live in developed countries.1‒5 Second, aging research suggested that the aging process per se might contribute to the toll of HF in the elderly aged ≥65years and older adults aged 45-64years.1 Third, translational research over the same period established that adverse cardiac remodeling plays a key role in the development and progression of HF and identified cardiac extracellular matrix (ECM) remodeling as a key underlying mechanism in both the development of HF and the subsequent march of adverse remodeling to end-stage disease in the aging patients.10‒14 Fourth, other translational research indicated that, after MI and HTN, divergent remodeling of the myocardium and cardiac ECM result in two distinct types of HF (Figure 1) – HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), respectively13‒16–suggesting that different or combined therapeutic strategies are needed to halt the progression to HF in aging patients.14
Despite these advances, proven therapies that specifically target cardiac ECM remodeling in HF in aging patients are lacking. This review focuses on the role of cardiac ECM remodeling in HF of older patients and some potential therapeutic targets.
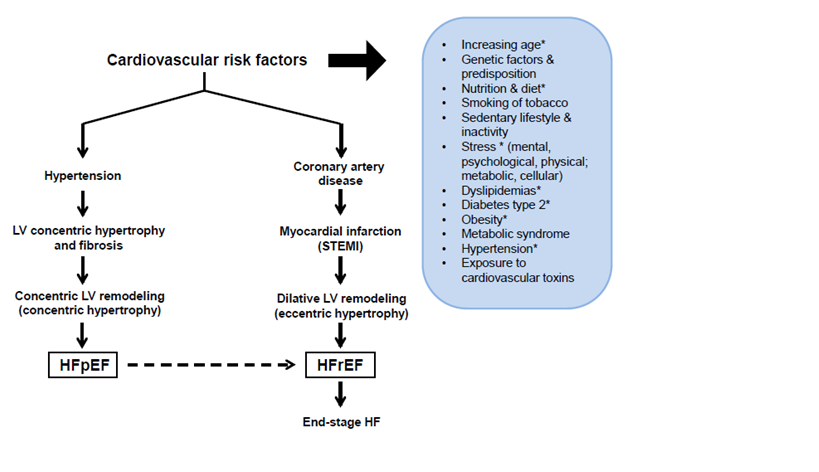
Figure 1 The two main types of cardiac remodeling and the march to heart failure in older patients. The two main co-morbidities in older patients, namely HTN and MI result in 2 divergent types of LV remodeling that lead to 2 distinct types of HF. The inset lists the main known CV risk factors. * Especially pertinent during the progressive aging process and subsequent march to HF.
EF: Ejection Fraction; HF: Heart Failure; LV: Left Ventricular; pEF: Preserved EF; rEF: Reduced EF; STEMI: ST-Segment Elevation Myocardial Infarction
Role of aging in cardiac and extracellular matrix remodeling
Biological aging is a progressive process. In the cardiovascular (CV) system, early research evidence suggested that aging results in progressive physiological, biological and structural changes that lead to increased ECM and fibrosis, increased ventricular-arterial stiffening, left ventricular (LV) diastolic dysfunction, and HFpEF6,7,12‒17 (Figure 2). A constellation of CV risk factors negatively impact the CV system throughout the aging process and fuel the adverse CV remodeling that leads to the development and progression of HF (Figure 3). In addition, the added insult of co-morbidities with aging further exacerbates and accelerates adverse remodeling and the march to HF (Figure 3)For optimal results, prevention strategies and therapeutic interventions need to separately target different incremental age groups and be evaluated in the different age groups in randomized clinical trials.18,19
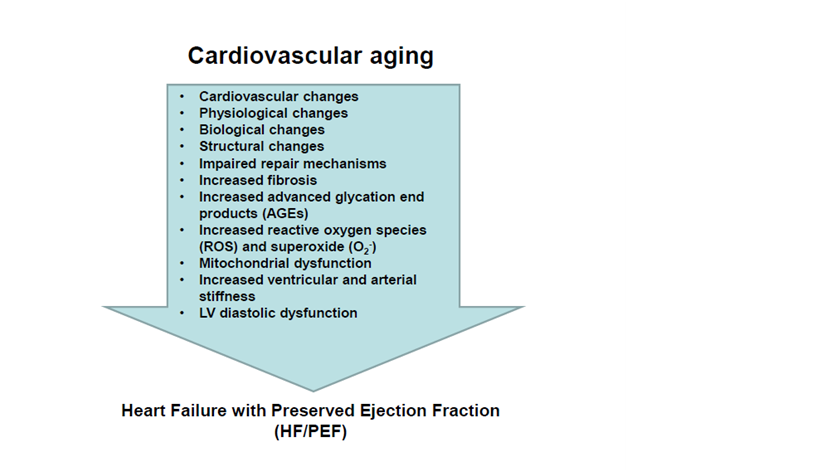
Figure 2 Cardiac and matrix remodeling during cardiovascular aging and heart failure with preserved ejection fraction. Insets list the main cardiovascular, physiological, biological and structural changes with aging.
: Increased; ¯: Decreased; EF: Ejection Fraction; HF: Heart Failure; LV: Left Ventricular; pEF: Preserved EF; rEF: Reduced EF; STEMI: ST-Segment Elevation Myocardial Infarction
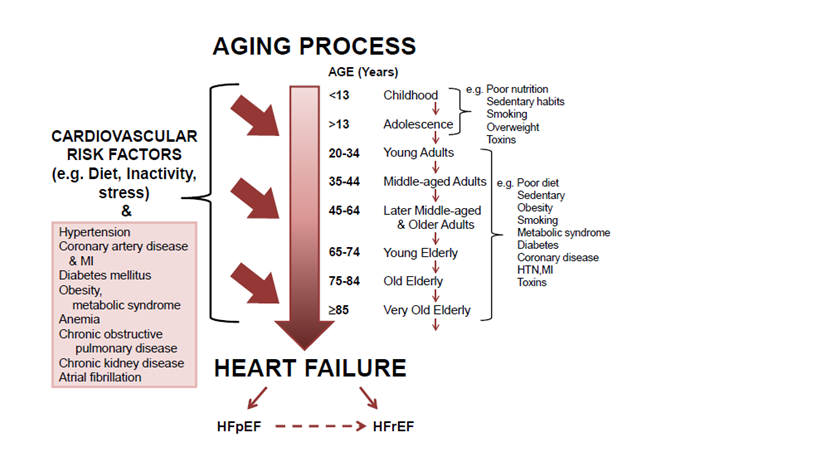
Figure 3 Prolonged exposure to risk factors and co-morbidities and the march to heart failure with aging.
A constellation of risk factors that impact the CV system throughout the aging process fuel adverse remodeling that in turn leads to progression and development of HF, and the added insult of co-morbidities further exacerbates and accelerates adverse remodeling. The changing substrate in different age groups emphasizes the need for different prevention/treatment strategies tailored to the respective incremental groups to ensure optimal results.
EF: Ejection Fraction; HF: Heart Failure; LV: Left Ventricular; pEF: Preserved EF; rEF: Reduced EF; STEMI: ST-Segment Elevation Myocardial Infarction
Cardiac and ECM remodeling after myocardial infarction
Cardiac remodeling following insults such as MI,10‒12,14,20‒32 HTN13 and various cardiomyopathies,3,16 involves progressive adaptive and maladaptive changes in cardiac structure, geometric shape and function that occur over time after the insult and lead to dysfunction.20‒25 Cardiac remodeling after MI and HTN each contribute to about 50% of all cases of HF16,19 and has been extensively studied in patient and experimental models of HF due to these conditions. During the early stage of an MI, damage of muscle, ECM and microvasculature is followed by a staged healing process, which through a timed sequence of biochemical, molecular and cellular/subcellular reactions over weeks, results in a fibrotic scar.10‒12,27‒30 Typically during post-MI healing, timed release of chemokines, cytokines, matrikines, growth factors including transforming growth factor-β (TGF-β), and matrix metalloproteinases (MMPs) and other matrix proteins occurs. The timed release of these proteins serves to orchestrate inflammation, remodeling of myocardium and ECM, and fibrosis.10‒12,27,28 Concurrent remodeling of cardiac structure and function is associated with complex biochemical, molecular, cellular and subcellular changes that further affect both cardiac muscle and ECM.10 Importantly, remodeling spans all the phases of the healing process and is progressive and extends well beyond.10‒12 During that time, multiple factors modulate remodeling of myocardium, ECM and vasculature post MI.10‒12 In post-MI survivors, the remodeling process extends to other cardiac chambers, tissues, cells and molecules, resulting in a vicious cycle leading to end-stage HF.32
After a large anterior transmural MI or ST-segment-elevation MI (STEMI), remodeling is dramatic and highly dynamic, leading to rapid progression to dilative LV remodeling with systolic dysfunction and HFrEF and poor outcome.17‒23,29‒31 Rapid early remodeling of the infarcted wall with infarct expansion (i.e. thinning and dilatation) is followed by progressive remodeling of the whole LV and a march to LV dilatation, dysfunction, volume overload, wall thinning, eccentric hypertrophy, HF, disability and death (Figure 1). While studies have shown that reperfusion of an occluded coronary artery within 30minutes after onset of an STEMI can limit infarct size, LV dilative remodeling and dysfunction, few patients can be reperfused that early. With the more common scenario of reperfusion being achieved ≥90minutes from the onset of STEMI, the result is usually adverse LV remodeling with persistent LV dysfunction and HF.31‒39 The causes of this paradoxical adverse remodeling and dysfunction with delayed reperfusion despite recommended medical therapy in management guidelines,16,34 include infarct size and reperfusion damage with no-reflow and flow-function mismatch at the microvascular level,12,18,#ref3030,32‒37 damage to the ECM,10‒12,29-32,39,40 and inflammation in early and late phases of healing.10‒12,30,38,41
Effect of aging on cardiac and ECM remodeling after myocardial infarction
Evidence suggests that, with aging, healing and repair of the damaged cardiac tissue after MI are impaired and result in augmented adverse remodeling affecting the entire left ventricle leading to HFrEF, as well as remodeling of the left atrium and right ventricle.7,12,18,32 Several factors in aging hearts, such as increased reactive oxygen species (ROS) and superoxide (O2-), and increased myocardial angiotensin II (AngII), which in turn, exerts pro-inflammatory, pro-oxidant and pro-remodeling effects that lead to increased pro-inflammatory cytokines, MMPs and oxidative markers, and thereby modulate healing and repair.31
Experimental studies
In a key experimental study of aging in young and old mice suggested that aging-related dysregulation of ECM and impaired healing after reperfused MI results in adverse LV remodeling.40,41 Several studies have shown that myocardial reperfusion after 90minutes of onset of MI is associated with an early increase in neutrophils and apoptosis that can augment myocardial damage 10-12,35. In a study of aging using the canine model of reperfused MI,31 120minutes of reperfusion after 90minutes of left anterior descending coronary artery occlusion was associated with increased pro-inflammatory markers such as TGF-b1, inducible-nitric-oxide-synthase (iNOS), cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-a, decreased anti-inflammatory markers such as neuronal NOS (nNOS), endothelial NOS (eNOS), and IL-10, and evidence of increased cardiomyocyte damage (ischemic injury, infarct size, apoptosis, blood flow impairment, and no-reflow), adverse LV remodeling (LV dilatation and dysfunction; increased LV volumes and asynergy; increased infarct thinning; increased left atrial pressure; decreased ejection fraction and deceleration time) as well as adverse ECM remodeling with increased expression of MMP-9 and MMP-2, increased ratios of MMP-9/tissue inhibitor of metalloproteinase (TIMP)-3 and MMP-2/TIMP-1 suggesting increased ECM turnover), and increased matricellular proteins such as secretory-leucocyte-protease-inhibitor (SLPI), secreted protein acidic and rich in cysteine (SPARC), osteopontin (OPN) and a disintegrin and metalloproteinase (ADAM) -10 and -17.
Studies in the rat model of reperfused MI also showed increase in these markers in the later phase of healing42,43 and the findings have been confirmed in the dog model.44,45 Taken together, these studies suggested that both early and late healing after reperfusion damage in older dogs lead to increased levels of matricellular proteins, proteolytic capacity, and pro-inflammatory activity in the infarct region; importantly, on the balance, aging appeared to be associated with impaired healing and augmented adverse ECM and cardiac remodeling and both systolic and diastolic dysfunction. In the same canine model, effects of AngII-blocker therapy with candesartan suggested that AngII modulates the age-dependent response to reperfused MI and the effectiveness of AngII-blocker therapy is blunted in older dogs.31,44,45 First, candestartan attenuated the age-dependent increase in infarct size and apoptosis but was less effective in the old than young.31 Second, candesartan normalized LV dilatation and systolic dysfunction but was less effective in the old than young. Reasons for this reduced effectiveness of AngII-blocker therapy in the old versus young include:
Clinical studies
In a key recent clinical study, Mahenke et al.,39 assessed ECM turnover in patients with a first-time STEMI who were successfully reperfused by primary percutaneous coronary intervention (PCI). They carefully analysed serial serum samples taken before PCI and at 2 and 7days, 2months and 1year after the STEMI, for markers of collagen synthesis and degradation, and their relationship with LV infarct size, ejection fraction, and volumes using serial cardiac magnetic resonance (CMR) imaging at the same time points. They documented that significant increase in collagen type I degradation following STEMI was not accompanied by increase in collagen type I synthesis until 2months and 1year. Importantly, in contrast to this delayed collagen type I synthesis, there was an immediate increase in collagen type III synthesis that was sustained for 1year. In addition, the N-terminal procollagen type I levels prior to PCI predicted adverse LV remodeling at all the CMR time-points. The findings indicated that net collagen type I degradation in the first week after STEMI was compensated by an early increase in collagen type III synthesis and subsequent increase in both collagen type I and III synthesis markers at 2months and 1year. Together, the findings suggested that collagen turnover is persistently increased after STEMI that is successfully reperfused by primary PCI and this persistent collagen turnover might participate in the adverse remodeling. Of note, the patients in that study were aged 58±12 (SD) years (i.e older adults and elderly).
Role of inflammation in matrix and cardiac remodeling post MI
Studies since the 1990s have addressed the mechanistic role of inflammation in matrix and cardiac remodeling after MI. Ten points deserve emphasis.
Role of MMP/ TIMP balance/imbalance in ECM and cardiac remodeling post MI
The cardiac ECM proteins, such as collagen, elastin, fibrillin, fibronectin, proteoglycans, and matricellular proteins, are finely organized in a complex 3-dimensional matrix assembly that ensure normal cardiac structure and function.10,11,62‒64 The organized interstitial network of collagen fibers provides the architectural support for cardiac myocytes, vascular channels and other cells, as well as the milieu for cell migration, growth, differentiation and interaction. An abundance of cardiac fibroblasts regulate ECM synthesis and deposition, mediate ECM degradation and turnover via MMPs and TIMPs, and maintain tension.62 Regulation of normal ECM remodeling with continuous ECM synthesis and degradation ensures maintenance of ECM homeostasis, cardiac shape and function via a fine balance between MMPs and TIMPs, and thereby prevents excessive ECM degradation that results in adverse remodeling.
The MMPs which represent the main proteolytic system for ECM degradation in the heart, are zinc-dependent endopeptidases that degrade ECM components such as collagens, fibronectin, proteoglycans, laminin and gelatin.10,11 Nearly 28 MMPs but only 4 TIMPS have been described and MMP activity is regulated post-translationally by the TIMPs.65 Four of the 5 classes of MMPs (collagenases, gelatinases, stromelysins and elastase) are secreted as latent pro-MMPs that bind ECM proteins and only become active upon cleavage of the propeptide domain via a cysteine switch mechanism involving serine proteases, trypsin, chymotrypsin and plasmin.65 Importantly, several MMPs can be activated by other pro-MMPs and trigger activation of more pro-MMPs leading to proteolysis. The fifth class of MMPs includes membrane-type MMPs (MT-MMPs) which are activated upon positioning in the cell membrane and retain the propeptide domain needed for activation and TIMP-binding.65 Injury triggers increased MMP expression, from basal levels in myocytes, fibroblasts, myofibroblasts and endothelial cells but also in inflammatory cells such as neutrophils, monocytes and macrophages. While most cells express TIMPs, TIMP-4 expression is highest in human myocardium. Besides inhibiting MMPs, the TIMPs are multifunctional and exert pro-growth, anti-apoptotic and anti-angiogenic effects.65
After MI, evidence indicates that LV remodeling is a main underlying mechanism for LV dilatation, HF and death, and ECM disruption is pivotal in dilative remodeling while AngII drives both ECM and LV remodeling.10‒12 A major pathway leading to ECM and dilative LV remodeling is through MMP/TIMP imbalance reflected in an increased MMP/TIMP ratio.10‒12 The main MMPs implicated in cardiac remodeling include collagenases MMP-1, MMP-8 and MMP-13, gelatinases MMP-2 and MMP-9, stromelysin MMP-3, and membrane-type MMP-14. After acute MI, a sharp rise in MMPs leads to rapid ECM degradation involving mainly fibrillary collagens in mature ECM (mostly cross-linked, thick and rigid type I collagen), and results in MMP/TIMP imbalance with increased ratio, adverse ECM and early LV remodeling with acute infarct expansion. This is followed by slow synthesis, deposition of immature, thin and elastic type III collagen, and subsequent slow maturation during healing/repair and beyond, resulting in further adverse LV remodeling.10‒12 This delayed replacement by rigid collagen type I and persistence of elastic collagen type III during healing/repair results in a vulnerable window of several weeks for adverse remodeling but also offers an opportunity for applying therapeutic interventions to limit remodeling.
Whereas MMP and TIMP levels subside over several days after acute MI, chronically higher MMP than TIMP levels can result in persistent ECM degradation and dilative LV remodeling, while higher TIMP than MMP levels can lead to increased ECM and fibrosis in the non-infarct zone resulting in diastolic dysfunction.
Six advances using genetic models support the aforementioned concepts and deserve mention. First, disruption of MMP-1, a collagenase from fibroblasts that has high affinity for fibrillar collagens and preferentially degrades collagen types I and III after MI,66 results in LV dilatation and dysfunction.67 MMP-1 synthesis is increased in MI67 and transgenic expression of MMP-1 inhibits fibrosis and the transition to HF in LV pressure overload.68 Second, deletion of TIMP-1, which is normally expressed by cardiac fibroblasts and myocytes and co-localizes with MMP-1 in myocardium, results in dilative remodeling post MI in mice.69 Importantly, increased TIMP-1 levels correlate with markers of LV remodeling and HF in patients with MI and HTN.69‒71 Third, both MMP-2 (gelatinase A) from myocytes, macrophages and myofibroblasts, and MMP-9 (gelatinase B) from neutrophils, macrophages, lymphocytes, cardiac myocytes, vascular smooth muscles cells, endothelial cells and fibroblasts have been implicated in post-MI remodeling.72‒74
Fourth, MMP-9 is somewhat unique in that it can process full length interstitial collagens as well as other substrates without the activation cleavage step for proteolysis, and not just collagen that has already been cleaved by collagenases such as MMP-1.75 MMP-9 also interacts with inflammatory response elements such as activator protein-1, specificity protein-1 and NF-kB and participates in the post-MI inflammatory response.75 Furthermore, both pharmacological inhibition and deletion of MMP-9 attenuate post-MI LV dilatation and dysfunction73‒76 while deletion stimulates angiogenesis in the infarct zone.76 MMP-9 levels correlate with inflammatory markers (such as IL-6, hs-CRP and fibrinogen) and CV mortality77 as well as with LV hypertrophy, MI, adverse remodeling with LV dilatation and dysfunction and HFrEF, and HFpEF.80 MMP-9 may therefore provide a prognostic biomarker for CV mortality.77 and adverse LV remodeling.78‒81
Fifth, aged TIMP-3 null mice show increased MMP-9, ECM degradation and LV dilatation, cardiomyocyte hypertrophy and LV dysfunction.82 TIMP-3 also regulates inflammation and inhibits ADAM-17 and -10 which can alter integrins (cell-surface matrix receptors), disrupt cell-matrix interactions, degrade ECM and contribute to LV dilation.83 These ADAMs also interact with inflammatory cytokines and alter MMPs, and thereby impact LV remodeling and/or injury.82 This finding underscores the fact that interactions between matrix proteins and inflammatory cytokines can modulate ECM remodeling.84 Sixth, while the role of MMP-3 (stromelysin-1) from myocytes remains unclear, elastase appears to modulate ECM degradation through activation of MMP-2, -3 and -9 and inactivation of TIMP-1,74 and elastase inhibition before reperfusion was shown to reduce infarct size.75
In summary, in survivors of MI and HTN, disruption of ECM or dysregulation of ECM homeostasis and metabolism augment adverse cardiac remodeling with shape deformation and dysfunction that result in HF, disability and death.10,84 A healthy cardiac ECM is therefore critical for preservation of cardiac shape and function, and adverse ECM remodeling plays a critical role in the march to HF.10,84
Cardiac and ECM remodeling in hypertension
Remodeling in patients with HTN progresses at a slower pace than with MI, and in response to chronic LV pressure overload results in concentric, hypertrophic and non-dilative LV remodeling; however, the latter may progress to LV dilative remodeling with eccentric hypertrophy and HF,7,26 which over time, may result in end-stage heart disease with congestive HF (Figures 1 & 3). As mentioned, a hallmark in hearts of hypertensive patients is excessive deposition of ECM with fibrosis,11,15 increased LV stiffness and typically HFpEF.11,15,16
In HTN, myocardial fibrosis which implies excessive ECM and collagen deposition, is mediated by well recognized mechanical and humoral mechanisms and leads to increased myocardial stiffness, LV diastolic dysfunction and HFpEF.81,85 Reactive fibrosis in HTN leads to increased stiffness86 and impared electrical activity.87 The regulators and suppressors of reparative fibrosis during healing/repair after MI have been reviewed,48 but the “braking” and “stop” signals in the infarct and non-infarct zones remains unclear. As discussed, net ECM degradation dominates the early phase of post-MI healing/repair and net ECM deposition, collagen synthesis and maturation dominate the later phase. In HTN, the “braking” and “stop” signals of reactive fibrosis also need study.
Collagen turnover and markers
As reviewed elsewhere,11,12 collagen turnover in cardiac ECM is regulated by fibroblasts and myofibroblasts. The fibroblasts and myofibroblasts synthesize and secrete preprocollagen type I and III as pro-α-collagen chains which form the triple helix structure of procollagens in the rough endoplasmic reticulum.11,88 Procollagen molecules are secreted from the Golgi complex into the interstitial space where cleavage of the end-terminal propeptide sequences allows collagen fiber formation. Post-translational modification of fibrillar collagen with addition of cross-links increases stability. Since specific procollagen N- and C-proteinases release the two terminal propeptides (i.e. amino (N)-propeptide and carboxy (C)-propeptide) of procollagen molecules into the circulation,89 the levels of these cleaved collagen propeptides can provide an indirect index of fibrillar collagen synthesis and deposition in patients with HTN.
Markers of synthesis include procollagen type I carboxy-terminal propeptide (PICP), procollagen type I amino-terminal propeptide (PINP), and procollagen type III amino-terminal propeptide (PIIINP).11,84 Serum PICP levels are elevated in both MI90. and diastolic HF,81 and correlate with the collagen fiber deposition in LV hypertrophy of spontaneously hypertensive rats.91 While PIIINP has been used as a marker of type III synthesis and to predict cardiac events and mortality, it may underestimate type III synthesis.92 Markers of collagen degradation include collagen type I carboxy-terminal telopeptide (CITP) and MMPs. High plasma CITP levels in post-MI patients correlate with poor outcome.92
Galectin-3 (Gal-3) which is expressed by activated macrophages and induces fibroblast proliferation and increased deposition of collagen type I and plays a role in the regulation of cardiac fibrosis and remodeling, has emerged as a potential marker of cardiac fibrosis in hypertrophied hearts.93 Post MI, Gal-3 expression is increased in the infarct zone of mice94 and in experimental and clinical HF84,95 but more studies in HTN are needed. Fibroblasts, which are the predominant non-myocyte cells in the heart and regulate ECM homeostasis in healthy and diseased hearts, play a critical role in fibrosis of MI, HTN and aging.62,95,96 Importantly, activated fibroblasts undergo phenotypic transformation into myofibroblasts that express contractile proteins, including α-smooth muscle actin, vimentin and desmin. Cardiac myofibroblasts are more sensitive to pro-inflammatory cytokines and hormones that are upregulated in remodeling hearts.97 Fibroblasts also act as “sentinel cells” that function as local immune modulators,98 and contribute to cardiac electrophysiology. It follows that cardiac fibroblasts and myofibroblasts may be potential candidates for optimizing healing/repair in post-MI survivors.
Therapy and modulators of post-MI remodeling and markers with aging
The modulators of post-MI remodeling and markers with aging have been reviewed.99‒101 As mentioned before, the majority of patients with HTN, MI and HF are older adults or elderly and old patients develop fibrosis and myocardial stiffness that result in diastolic dysfunction with HFpEF even in the absence of MI or HTN. Importantly, specific therapy for HFpEF is lacking. Clinical studies that have tested inhibitors of collagen synthesis for limiting cardiac fibrosis have failed. Encouraged by successful attenuation of post-MI LV remodeling with broad-spectrum as well as selective MMP inhibitors in post-MI mice and pigs, and chronic HF patients,102 a larger clinical study of MMP inhibition initiated after acute STEMI failed to limit LV remodeling.102
Renin-aldosterone-angiotensin system (RAAS) inhibitors such as angiotensin-converting enzyme (ACE) inhibitors, AngII-type 1 receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs) can all limit fibrosis10‒12 and combination therapy is often necessary in the elderly. However, their use in patients with MI and HTN has not prevented the march to HF. The attractive idea of a dual-action molecule that targets both natriuretic peptide and AngII pathways was tested with LCZ696, which combines neprilysin and the ARB valsartan. LCZ696 was shown to benefit patients with HFpEF in a phase II trial103,104 and is being evaluated in patients with HFrEF in a phase III trial.103,104 Since is associated with RAAS remodeling,105 whether dual pathway inhibition and other RAAS and novel therapies may be more effective in elderly HF patients needs study.
Aging and cardiac ECM remodeling
The traditional view is that four main features make up the aging phenotype, and these are increased concentric remodeling, increased mass to volume ratio, increased ECM (fibrillary collagen content), and decreased diastolic function and relaxation.14,17 Ten points in aging research that support the current concepts need emphasis. First, aging-induced ECM remodeling begins with ECM regulation and collagen synthesis, deposition, maturation and degradation. Increased deposition is dependent on interactions among signaling pathways, protein synthesis, post-translational modification and enzyme activity. Second, aging induces progressive loss of myocytes and myocyte hypertrophy,106,107 left atrial dilatation and atrial fibrillation,108 and results in HFpEF.109 Third, findings in the senescence-accelerated mouse support the idea that cardiac fibrosis leads to LV diastolic dysfunction and depressed compliance independent of vascular stiffening.110 Compared to age-matched senescence-resistant mice, 6-month old senescence-accelerated mice showed reduced left-atrial/LV filling ratio, increased end-diastolic pressure and diastolic stiffness, and LV fibrosis but no change in ventricular/vascular coupling ratio, ejection fraction or LV size.110 Fourth, naked mole rats with long life spans,111 old male rats show no change in left-atrial/LV filling ratio whereas old female rats develop diastolic dysfunction.112
Fifth, increased cross-linking of amino groups in fibrillar collagen induced by reducing sugars that yield advanced glycation products (AGES) is seen with aging and pathological remodeling.113,114 Interestingly, SPARC binds to protocollagen and mediates mature cross-linking,115 and SPARC ablation results in age-dependent decreased collagen content and cross-linked collagen, and decreased fibrosis with pressure overload.116,117 SPARC null mice have been shown to develop defective scars and cardiac rupture after MI.118 Sixth, with regard to ground substance, aging has been reported to either decrease119 or have no effect on glycoaminoglycans (GAGs),120 increase the non-sulfated GAG hyaluronan (hyaluronic acid) and decrease chondroitin sulfate,120 and increase the GAG heparin sulfate.121 Seventh, aging results in increase in the adhesive protein fibronectin in pressure and volume overload and MI.122‒125
Eighth, aging leads to cardiac fibrosis even in the absence of co-morbidities. Several studies have shown decreased myocardial collagen synthesis with aging, suggesting post-translational modification and/or decreased degradation.126‒129 In contrast, collagen synthesis increases in HTN and pressure-overload hypertrophy.130‒132 Ninth, the latest of the MMPs, MMP-28 (epilysin) is expressed constitutionally in macrophages of adult hearts, and is increased in older mice but deletion amplifies the inflammatory and ECM responses with aging1333 and augments dysfunction and rupture post MI.134 Tenth, in sheep with pacing-induced heart failure, older sheep aged 8years show decreased LV collagen content, increased MMP-2 and decreased TIMP-3 and -4, and increased SPARC compared to younger sheep aged 1.5years.135 However, serum PICP was increased in both age groups.135
Recent advances towards developing therapy for HFpEF
The previous discussion has focused on the role of cardiac ECM remodeling in HF of older patients and some potential therapeutic targets. The two main types of adverse remodeling and HF encountered in the elderly population (HFpEF and HFrEF) have been underscored. The concept of lifelong exposure to a constellation of CV risk factors throughout the aging process in fueling adverse remodeling that in turn leads to progression and development of HF, and the role of the added insult of co-morbidities in exacerbating and accelerating adverse remodeling with aging have been emphasized (Figures 1-3). The lack of specific therapy to limit aging-induced adverse remodeling and the march to HFpEF in 2015 underlines the need for more research.
A recent report on developing therapies for reducing hospitalization and mortality in patients with HFpEF patients hosted by the Food and Drug administration Bureau in the United States recognized the increasing prevalence of HFpEF, increasing risk for adverse outcomes, the lack of specific approved therapy, the lack of animal models for the study of HFpEF, the need to understand the role of comorbidities, and the multiplicity of potential CV structural targets, mechanisms and pathways.136 The participants endorsed better understanding of pathophysiological pathways and better identification of therapeutic targets.136 While they debated whether HFpEF represents a collection of comorbidities, the importance of CV changes with aging was not highlighted in the report.136
Of note, in the HFpEF trials, the LVEFs were >40-50%, mostly > 45%, and fewer with LVEF > 40% (i.e. the lower range).136 In trials with the ARB irbesartan in HFpEF137,138 and the ACE-inhibitor candesartan in HF137,139 showed lower CV mortality (70%) in HFpEF137,139 and higher CV mortality (83%) in HFrEF.137,139 Trials with ACE-inhibitors, ARBs, digoxin, beta-blockers, spironolactone and sildenafril did not lower mortality in HFpEF while results with LCZ696 are awaited.136 Importantly, Campbell et al.,137 suggested that HFpEF extends beyond age and comorbidities. Paulus et al.,140 recently underscored the role of systemic inflammation related to comorbidities and postulated a cascade of 5 steps leading to HFpEF. In their construct, the high prevalence of comorbidities (such as overweight/obesity, diabetes mellitus, chronic obstructive pulmonary disease, and salt-sensitive HTN) induce a systemic pro-inflammatory state which causes coronary microvascular endothelial inflammation, that in turn reduces NO bioavailability, cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG) activity in adjacent cardiomyocytes. The low PKG activity in turn favors hypertrophy and increased resting tension due to reduced phosphorylation of titin. Finally, the stiff cardiomyocytes and interstitial fibrosis contribute to high diastolic LV stiffness and HFpEF.140 They suggest that diagnostic algorithms should include comorbidities, plasma markers of inflammation or vascular hyperemic responses and therapy should aim at restoring myocardial PKG activity.140 Whether co-morbidities might explain the failure of previous trials in HFpEF is unclear.
A recent study of elderly adults (mean age 73-74years) showed that adverse LV remodeling predicts incident HF and mortality.141 The majority of the patients had EF ≥ 45%. The authors used a combinatorial assessment of LV chamber size and wall mass to identify older adults with HF and concluded that the addition of relative wall thickess (RWT or ratio of septal plus posterior wall thickness to end-diastolic dimension by echocardiography) to the analysis of LV remodeling and outcome only had incremental value when the LV is not enlarged.141 In a previous report on mostly older patients, the majority of patients with HFpEF showed LV hypertrophy or concentric remodeling, left atrial dilatation, and diastolic dysfunction.142 Other biomarkers of HFpEF have been reviewed elsewhere.99,100,143 The biomarkers that appeared most promising included those of myocyte stress, inflammation, ECM remodeling, growth differentiation factor 15 (GDF-15), cystatin C, resistin, and galectin-3.143 Soluble ST2 is considered a prognosis biomarker in HF and is involved in multiple pathways including cardiac strain, inflammation, necrosis and remodeling.144 The new biomarker ST2 belongs to the IL-1 superfamily involved in inflammation, and increased soluble ST2 in the circulation acts as a decoy receptor for IL-33, thereby blocking its antinecrotic and antiremodeling effects, and promoting fibrosis and ventricular dysfunction.144
Acronyms for the types of HF
It should be noted that the literature on the use of the acronyms for the 2 major types of HF in older patient populations has been evolving and is still being debated. Paulus has used HFPEF and HFREF,15,16,140 as have the past ESC and AHA/ACC guidelines.3‒5 Recent publications favor the acronyms HFpEF and HFrEF136 and these are used here.
Cardiac remodeling is multifactorial, highly dynamic and time-dependent. Dysregulated ECM homeostasis in survivors of MI and HTN leads to adverse ECM and cardiac remodeling, LV dysfunction and poor outcome. Defective ECM and fibrosis with increased cross-linking can augment adverse LV remodeling and systolic/diastolic dysfunction. Decrease in ECM and fibrosis and/or defective ECM (with increased collagen type III and decreased or abnormal cross-linking) can lead to more adverse LV remodeling with predominant systolic dysfunction and even LV rupture. After STEMI, combined damage of muscle, ECM, and microcirculation and not just ECM drives dilative LV remodeling with HFrEF. In HTN, combined LV hypertrophy and excess ECM/fibrosis drives concentric LV remodeling with HFpEF, and later mixed LV remodeling with HFrEF. Despite divergent types of remodeling post MI and HTN (i.e eccentric versus concentric; Figures 1 & 3), they may converge due to changes with aging and other co-morbidities (Figure 3).
Importantly, despite conventional therapy, both types of remodeling and HF progress to end-stage HF, disability and death (Figures 1 & 3). Sound knowledge of modulators and markers of matrix remodeling is therefore critical in efforts to identify targets to prevent, interrupt/limit/reverse the progression to end-stage HF99,100,143 for review. It is important to remember that therapy with RAAS inhibitors and beta-blockers decrease ECM and may be beneficial in HTN and remote MI but the benefits may be blunted with aging. Identifying a specific therapeutic target may be made difficult because multiple pathways are often involved and co-morbidities with more pathways are often involved. For optimal benefit in elderly patients, future strategies may need combined approaches comprising novel substrates, targets and regimens, mechanical approaches, and matrix-tissue engineering, regeneration and transplants.84 Efforts to prevent, interrupt, limit or reverse the march of cardiac remodeling to end-stage HF in any age group should first be to identify key modulators, early markers, and potential therapeutic targets in post-MI and post-HTN remodeling processes in aging patients. More translational and clinical research into cardiac aging and testing of therapies in aging animal groups and elderly patient groups is needed.
I am indebted to Bernadine A Jugdutt for expert assistance with the figures.
Author declares there are no conflicts of interest.
Author declares there are no conflicts of interest.

©2015 Jugdutt. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.