Journal of
eISSN: 2373-4396


Research Article Volume 4 Issue 1
1Michael G. DeGroote School of Medicine, McMaster University, Canada
2Department of Clinical Epidemiology & Biostatistics, McMaster University, Canada
3Division of Pediatric Cardiology, McMaster University, Canada
Correspondence: Tapas Mondal, Associate Professor Pediatrics, division of Cardiology, McMaster University, 1200 Main Street West, room number 3A54 , L8N3Z5 Hamilton, Ontario, Canada, Tel 9055212100, Fax 9055211703
Received: November 15, 2015 | Published: November 23, 2015
Citation: Mondal T, Khan R, Ranganathan M. Accuracy of the electrocardiograph in detecting cardiac left ventricular hypertrophy in pediatric patients with aortic valve disease. J Cardiol Curr Res. 2015;4(1):1-6. DOI: 10.15406/jccr.2015.04.00129
Background: Left ventricular hypertrophy (LVH) is a pathological remodeling of myocardial tissue occurring in response to an increase in cardiac workload. The most significant cause of LVH in the pediatric population is aortic valve disease, which includes aortic valve stenosis and insufficiency. Though LVH can arise naturally in response to strenuous exercise, it has clinical utility as a marker of hypertension and heart disease. LVH can be detected using certain voltage criteria via electrocardiogram (ECG), though this approach typically exhibits poor sensitivity. The current gold standard for LVH identification is echocardiogram – however, waiting times and operator skill are significant variables in echocardiographic diagnosis of LVH. This study evaluates the sensitivity and specificity of various voltage criteria for LVH in this population, and whether amalgamating criteria can boost their accuracy.
Methods: A retrospective chart review was conducted to look at the echocardiograms and electrocardiograms (ECGs) of 62 pediatric patients with aortic valve disease seen at McMaster University Medical Centre. Patient ECGs were analyzed for LVH using a number of voltage criteria: those established by the Journal of American College of Cardiology’s (JACC) standards for pediatric LVH, criteria utilized for adult LVH, and Cornell adult voltage criteria. To determine the suitability of these indices in identifying LVH via ECG, we analyzed sensitivity, specificity and negative predictive value (NPV) of each set of criteria using echocardiograms carried out within 2months as a gold standard.
Results: Our results identify Cornell Female voltage criteria as bearing the highest sensitivity (81.94%) in identifying LVH in this population, while JACC pediatric criteria demonstrated the highest specificity (77.29%). Using Cornell Female voltage criteria as a sensitive screening tool, we then applied JACC pediatric criteria (chosen for its high specificity). The combined criteria (JACC-CF) demonstrated higher sensitivity and specificity (88.64% and 83.33%, respectively) than either criteria used in isolation.
Conclusion: Cornell Female voltage criteria demonstrated the highest sensitivity in this population, though it is typically not utilized in a pediatric setting. Utilizing Cornell Female criteria as a screening tool in tandem with JACC criteria for its specificity yielded a set of highly specific and sensitive criteria. This approach bears promise in pediatric patients with aortic valve disease, and should be explored in other populations with LVH.
Keywords: left ventricular hypertrophy, electrocardiogram, aortic valve disease, sensitivity, specificity
LVH, left ventricular hypertrophy; LVD, left ventricular dilation; AVD, aortic valve disease; ECG, electrocardiogram
Left ventricular hypertrophy (LVH) is a thickening of myocardial tissue of the left ventricle (LV) in the heart.1 Changes associated with LVH include cardiac fibrosis and remodeling, as well as cardiomyocyte hypertrophy.2 LVH typically arises as a compensatory mechanism against a chronic increase in cardiac workload, though neurochemical and hormonal stimuli have also been implicated, including mediators such as cardiotrophin – 1 and parathyroid hormone.3‒5 These mechanical stresses placed on the heart may be pressure or volume-mediated.6 Pressure-induced hypertrophy induces concentric hypertrophy (thickening of cardiac muscle without dilatation), while volume-mediated hypertrophy is termed eccentric hypertrophy, or left ventricular dilatation (LVD).6 The difference between these forms of hypertrophy stems from the addition of sarcomeric units added in series (eccentric) or in parallel (concentric).2 A moderate level of LVH is observed to arise naturally in response to exercise, but even this can progress to a condition known as athlete’s heart where individuals demonstrate some risk for adverse cardiac events.7 Initially, the increase in left ventricular wall mass is designed to ameliorate wall stress, but eventually becomes maladaptive. As a result, LVH’s most apparent role is as a clinical marker and predictor of unfavourable cardiac outcomes, second only to age.8‒10
Pediatric patients with aortic valve disease (AVD) can present with varying degrees of pressure and volume-mediated stresses upon the left ventricle, with a particularly prevalent example being aortic stenosis.11,12 Aortic stenosis refers to a narrowing of the aortic valve, resulting in greater resistance (by Laplace’s law) faced by the left ventricle. Over time, this can translate into a maladaptive burden and an increased afterload upon the left ventricle, leading to the development of LVH as a compensatory mechanism.6,12 These ventricular modifications may be detected through voltage changes – the 12-lead electrocardiogram (ECG) is an important tool in preliminary detection of left ventricular voltage changes. A variety of indices may be applied to ECG lead voltages, including the Sokolow-Lyon/JACC (SV1+RV5 or SV1+RV6) or Cornell (SV3+RaVL) threshold voltage criteria for LVH (Table 2). However, these criteria typically lack sensitivity, and accordingly the utility of ECGs in LVH screening has been called into question.13 Reported ECG sensitivities range from under 20% in HIV-positive children14 to 67% in patients with aortic stenosis or septal defects.11 As a result, echocardiograms must typically be used to confirm or validate ECG voltage abnormalities. However, wait times for echocardiograms can be lengthy, with mean wait times in British Columbia approximated at 10.7 ± 6.1weeks.15 By comparison, ECGs are inexpensive, non-invasive and may be conducted without an extensive set of instruments or specialist involvement.
The present study’s interest is in improving the utility of the ECG with respect to LVH and LVD detection in pediatric patients with AVD. Combining ECG criteria has been shown to increase the sensitivity of LVH detection,16 but this approach has not been characterized in our cohort of interest. Augmenting the sensitivity of ECGs, which are already highly specific, would increase their utility in diagnosis and allow for a more judicious use of echocardiography as an LVH screening tool. Using echocardiographic measurements as a gold standard, we report the sensitivities, specificities and negative predictive values associated with four separate ECG criteria for LVH: Adult voltage criteria, JACC-Age related criteria, Cornell Adult Female criteria and Cornell Adult Male criteria. These were examined in the context of patients with LVH, LVD, and those with both conditions. We also applied the Cornell Adult Female voltage to ECG events positive for LVH by JACC-Age related voltages, and demonstrated a considerable increase in sensitivity using this method. We report that combining ECG voltage criteria does yield a detection method of LVH voltages with higher sensitivity in pediatric AVD patients. Further investigation and reevaulation of the electrocardiogram is warranted, particularly with respect its use as a screening tool in left ventricular hypertrophy.
Patient records
A retrospective chart review was conducted of 61 pediatric patients (Figure 1) with aortic valve disease seen at McMaster University Medical Centre between 2005-2013. Access to Meditech for retrieval and survey of patient records was granted by the McMaster Research Ethics Board.
|
Criteria |
Sensitivity |
Specificity |
Negative predictive value (NPV) |
|
Adult |
68.6 |
70.57 |
88.66 |
|
JACC Age-related |
69.41 |
77.29 |
89.03 |
|
Cornell Adult Female |
81.94 |
62.24 |
94.2 |
|
Cornell Adult Male |
65.55 |
73.52 |
87.2 |
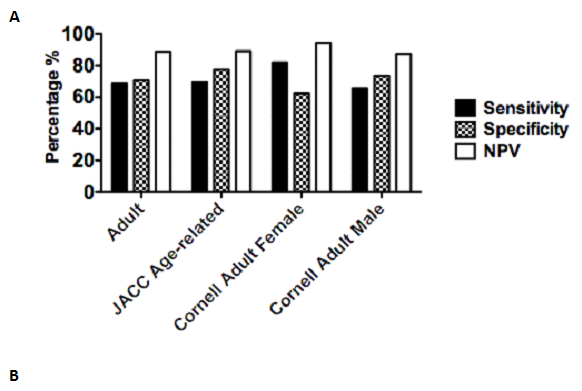
Figure 1 Sensitivity, specificity and negative predictive values of ECG LVH voltage criteria.
Records were obtained from 61 pediatric cardiology patients at MUMC, and their ECG voltages were examined using four separate voltage indices for LVH and compared to the results of a corresponding echocardiographic test. Echocardiography performed within 2 months of the ECG was used as a gold standard. Values are representative of 270 events between 61 patients. Sensitivity, specificity and NPV were calculated using patients positive for either LVH and/or LVD by echocardiography. Results are displayed in (A) graph and (B) chart format. The highest value in each category is bolded.
Electrocardiography/echocardiography records
Patient ECGs were examined for LVH according to the Journal of American College of Cardiology (JACC) pediatric standards using Sokolow-Lyon criteria, defined as: SV1 + RV6, with values stratified by pediatric age bracket or adult criteria.17 Cornell adult voltage criteria were also used to check for LVH, defined as: SV3 + RaVL> 20 in females and SV3 + RaVL> 28 in males.18 Indices and threshold voltages for the detection of LVH using electrocardiography are summarized in Table 2. ECGs were matched to echocardiograms within 2 months. LVH was identified from the echo reports on the basis of interventricular septal thickness & posterior wall thickness z scores ≥ 2 or LV mass index z scores ≥ 2, while LVD was identified when LV end diastolic dimension z scores ≥ 2.
Statistical analysis
Sensitivity, specificity and negative predictive values (NPVs) were calculated for all ECG indices (Table 2). Echocardiography was used as a gold standard, of which echocardiograms showing evidence of LVH and/or LVD were considered true positive events. Statistical analysis was conducted using Microsoft Excel 2011 and GraphPad Prism 5.0.
Patient demographics and electrocardiographic voltages
The records of 61 pediatric patients seen at McMaster University Medical Centre from 2005 to 2013 were examined. The population age ranged from 3months to 18years. Patient ages are reported as of April 2013 – however, ECGs have been assigned to categories reflecting the age at which patients were seen at McMaster University Medical Centre. A total of 270 ECGs matched to echocardiograms were selected for voltage evidence of LVH according to Sokolow-Lyon and Cornell criteria.17 Though 295 ECGs were gathered from participant medical history, 25 ECGs were excluded, as a corresponding echocardiogram within 2months was absent, preventing us from using our gold standard accurately.
Our aim was to examine the efficacy of ECGs in identifying LVH through voltage alone. As previously mentioned, LVH voltage detection through ECG has been shown to lack sensitivity in a number of disease contexts.11,14 We were interested in the utility associated with 4 separate indices for voltage detection. We chose age-dependent JACC criteria and JACC adult criteria, both of which utilize the Sokolow-Lyon/JACC sum of SV1 and RV6, but with differing threshold voltages. In addition, we also examined Cornell adult male and female voltages, which use the sum of leads RaVL and SV3 and are not traditionally applied to pediatric populations. ECG voltage criteria with patient averages for lead voltage sums are recorded in Table 2.
|
Variable |
Value |
|
Number of patients: |
61 |
|
Male |
50 |
|
Female |
11 |
|
Age Range: 3 months-18 years: |
|
|
7 days-1 year |
6 |
|
1-3 years |
4 |
|
3-5 years |
9 |
|
>5 years |
42 |
|
Total number of ECG-echo events: |
270 |
|
7 days-1 year |
47 |
|
1-3 years |
31 |
|
3-5 years |
29 |
|
>5 years |
163 |
|
Average number of ECG-echo events/ patient: |
4.43 |
|
Echocardiography |
|
|
Events showing LVH only |
39 |
|
Events showing LVD only |
91 |
|
Events showing LVH & LV dilatation |
20 |
Table 1 Patient demographics including gender and age distribution, and number of ECGs and echocardiographs
|
Criterion |
Threshold (mm) |
Patient Averages |
|
Adult Criteria (Sokolow-Lyon): SV1 + RV6 |
>35 |
34.30 ± 14.09 |
|
JACC Age-related Criteria (Sokolow-Lyon): SV1+ RV6 |
||
|
7 days-1 year |
>35 |
23.20 ± 10.99 |
|
1-3 years |
>38 |
30.30 ± 9.48 |
|
3-5 years |
>42 |
31.50 ± 11.54 |
|
>5 years |
>47 |
39.10 ± 13.82 |
|
Cornell Adult Male: RaVL + SV3 |
>28 |
|
|
Cornell Adult Female: RaVL + SV3 |
>20 |
25.2 ± 10.55 |
Table 2 Criteria used to evaluate LVH from ECG lead voltages, including patient lead averages
Cornell Adult Female threshold voltages demonstrate the highest sensitivity for LVH detection in pediatric AVD patients
To study ECG utility in detection of concentric or eccentric LVH (LV dilatation), we examined sensitivities, specificities, and negative predictive values (NPV) associated with various ECG voltage indices (Figure 1). We considered true positive events as patients found to have either LVH and/or LVD by echocardiography. In this context, we found that the JACC age-dependent threshold voltages yielded the highest specificity (83.83%), while Cornell adult female voltages yielded the highest sensitivity (81.76%). Cornell adult female voltages also demonstrated the highest NPV (82.84%). We also recognized that we were able to find more ECGs for some patients than others, and were concerned that these individuals were overrepresented in our sample. To account for this, we analyzed only the earliest ECG available for each patient and calculated sensitivity, specificity and negative predictive values utilizing our four ECG voltage criteria (Figure 2).
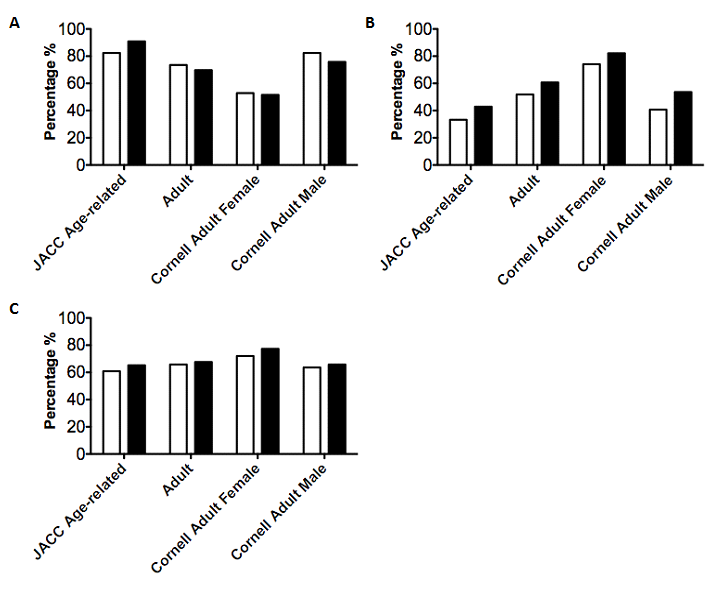
Figure 2 Sensitivity, specificity and negative predictive values of LVH criteria generated from a patient’s first and last available ECG.
ECG voltages were examined using four separate voltage indices for LVH and compared to the results of a corresponding echocardiographic test. Echocardiography within 2 months of an ECG was used as a gold standard to determine the presence of anatomical LVH. Data was only used from the first available ECG for a patient (First Visit) and the last available ECG for a patient (Last Visit). Sensitivities (A), Specificities (B) and NPVs (C) were calculated using patients positive for LVH and/or LVD via echocardiography.
Amalgamating ECG criteria with high sensitivity (Cornell Adult Female) and high specificity (JACC Age-related) result in improved ECG concordance in detection of LVH
Our next scenario of interest involved amalgamating ECG voltage criteria. As Cornell Adult Female criteria exhibited the highest sensitivity, we decided to use this criterion as a screening tool. We then applied JACC Age-related criteria (bearing the highest specificity) to these events and analyzed sensitivity, specificity and NPV using to LVH and/or dilatation by echocardiography as our gold standard. Our amalgamated JACC+CF criteria showed a sensitivity of 88.64%, a specificity of 83.33% and a negative predictive value of 89.47% (Figure 3A). These were higher than either JACC or CF criteria alone, with the exception of JACC criteria specificity.
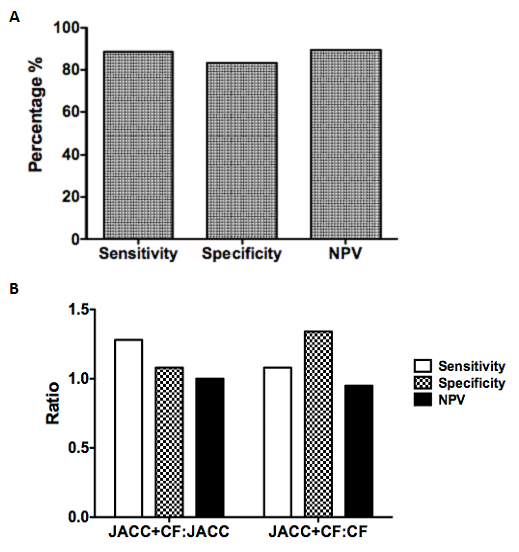
Figure 3 Analysis of amalgamated criteria.
ECGs indicating LVH by Cornell Female voltages were then analyzed using JACC-Age related voltages. These data were compared against corresponding echocardiographic data within 2 months as a gold standard using events positive for LVH and/or LVD and analyzed to calculate sensitivity, specificity and negative predictive value. Additionally, amalgamated criteria were compared to individual criteria alone in a ratio format (B). Data represents 95 matched ECG-echocardiograms among 61 patients, which indicated the presence of LVH by JACC-Age related voltages.
This study’s interest lies in comparing the sensitivity and specificity of commonly utilized ECG threshold voltages for LVH, as applied to a pediatric population with aortic valve disease. As previously mentioned, ECGs lack LVH detection sensitivity in a number of different disease contexts.11,14,19‒22 This may stem from the fixed nature of threshold voltage criteria, which are subject to demographic variability in gender, body weight and age among others.22 We observed that ECG detection of LVH in the pediatric population proved most sensitive through utilizing voltage criteria from Cornell Adult Female criteria. This was most pronounced in the detection of patients who had hypertrophy and/or left ventricular dilatation. The sensitivities of the other criteria were lower, in the order of Adult criteria (71.04%), JACC Age-related (67.71%) and Cornell Male criteria (67.01%). Sensitivity of ECGs in detection of pediatric LVH is reported at 25.3%.23 However, Fogelet al. report LVH criteria sensitivity at 67% in pediatric patients with aortic stenosis, though this study relied on JACC Adult criteria alone (SV1 + RV6) and had a low number of patients.11
For calculations of sensitivity and specificity, we used patient echocardiograms as the gold standard. Cardiac magnetic resonance imaging (MRI) has been shown to be a more sensitive measure of in vivo heart function in both small animals24 and human patients,25 where echocardiography is reported to underestimate atrial and ventricular dimensions but overestimates wall thickness.26 However, our patients did not receive cardiac MRI, which led to us choosing 2-D echocardiography as our gold standard. It is possible that cardiac MRI may have led to a more accurate evaluation of ECG voltage criteria – though given the ubiquity of echocardiography we believe our approach to be a more realistic marker by which to measure ECG sensitivity and specificity. Echocardiography has its own set of logistical issues, however. As mentioned before, wait times for echocardiography are estimated at 10.7weeks in British Columbia,15 but similar data are not available for the rest of Canada. Additionally, echocardiography demonstrates a degree of operator dependence,25 while electrocardiograms may be conducted with minimal specialist involvement.
A further point of discussion is the comparatively low number of female11 versus male patients (50)– given the male overrepresentation, more study is necessary to see how well these approaches perform in a balanced study sample. Additionally, the adult female heart generates less voltage as a result of its lower wall mass,27 and it is possible these voltage differences may persist in the pediatric setting. However, it is unclear if a specific criterion is superior in LVH detection for a particular gender, and different studies offer different results. Investigations of Spanish and UK patients with cardiovascular disease and untreated hypertension respectively suggest that Sokolow-Lyon voltages offer superior correlation with LVH in men.28,29 However, an investigation utilizing cardiac MRI as a gold standard indicated that Cornell voltages were superior for males, while Sokolow-Lyon criteria were superior for females.30 Evidence regarding gender disparities with respect to LVH detection by ECG in pediatric populations is scarce. An investigation by de Simone et al. suggests that differences in left ventricular wall mass are minor in pre-adolescent patients, but that during adolescence male patients exhibit a higher rate of left ventricular growth than females.31 Accordingly, Rijnbeeket al. report gender differences in ECG voltage amplitudes and QRS duration for children aged 12-17years.32 These data indicate that some disparities do exist in ECG presentation between genders in the pediatric population, but further investigation is needed with respect to pediatric AVD patients.
Additionally, a number of our patients had more ECGs and echocardiograms available than others – we investigated whether overrepresentation of these patients may have affected our metrics. To address this issue, we applied our 4 ECG voltage criteria to only the first and last ECGs from each patient. Cornell Female criteria remained the most sensitive in both first and last visits, but the hierarchy of the other criteria changed. However, this corroborates the novelty of this study – the high sensitivity of Cornell Adult Female criteria may hold clinical relevance with regard to LVH diagnosis in this population, as this criterion is not typically employed in pediatric ECGs. However, Brothers et al. recently utilized Cornell voltages in patients aged 7-21 with hypertrophic cardiomyopathy, but did not use the Adult Female or Adult Male criteria for all patients, as we have done.33 Both Cornell male and female criteria demonstrate greater sensitivity than Sokolow-Lyon voltages in detecting LVH among adult Koreans and patients with previous anterior myocardial infarction.34,35 Brothers and colleagues also employed a novel voltage index of aVL+SV2, with a sum greater than 23mm chosen to indicate hypertrophy33 – it remains to be seen how effective this criterion is in pediatric AVD patients.
As ECGs have proven to be an ineffective screening tool for other cardiac pathologies, we investigated whether pairing criteria being high sensitivity with criteria bearing high specificity would produce an improved LVH detection approach with both high sensitivity and specificity. First, we applied Cornell Female criteria (chosen for its high sensitivity in the full set of ECGs) to patient ECGs, and determined which events were positive for LVH. We then applied JACC Age-related voltage (chosen for its high specificity) criteria to electrocardiograms positive for LVH via Cornell Female criteria. This led to an increase in sensitivity (88.64% vs 81.76% with Cornell Female), and specificity (83.33% vs 77.29% with JACC Age-related). These data indicate that applying multiple voltage criteria can indeed generate a more sensitive and specific approach in pediatric patients with aortic valve disease, and a similar approach may be possibly for other cardiac abnormalities in other populations. Additionally, data indicate that Cornell and Sokolow-Lyon voltages for LVH may identify different segments of adult populations, especially in the case of patients with cardiovascular disease28 and hypertensive patients taking losartan.36 Hypertensive individuals showing electrocardiographic LVH by Cornell criteria were associated with factors such as increased age, female gender, obesity, and diabetes mellitus while individuals with hypertrophy by Sokolow-Lyon were associated with younger age, male gender, angina and cerebrovascular dysfunction. Usage of these criteria in tandem, as we have done in this study, may capture different segments of the pediatric population as well and explain the rise in sensitivity.
Our work further elucidates the utility of various ECG voltage criteria for LVH in a pediatric population with aortic valve disease. We demonstrate that these criteria offer both high specificity and sensitivity in this population, and refining threshold voltages may improve their performance in detecting LVH. We conclude that ECG voltages may be useful clinically in detecting LVH in pediatric AVD patients and have high concordance with echocardiogram findings. Using a sensitive voltage criterion (Cornell Adult Female) as a screening test, followed by a specific criterion (JACC Age-related) resulted in an approach that was both sensitive and specific in pediatric patients with aortic valve disease. This may also offer an additional tool for physicians to evaluate LVH in pediatric AVD patients in a primary care setting, as electrocardiograms are routinely ordered and preliminarily evaluated by primary care providers. Judicious use of sensitive ECG criteria for cardiac lesions may lower the use of unnecessary echocardiograms and lower healthcare costs for the province and taxpayer. Future study is needed to characterize this approach in this population and others, and how this relatively inexpensive diagnostic can be further improved.
Medical writing services were provided by Dr. Andreas Völp, Psy Consult Scientific Services, Frank furt, Germany. This work, including provision of all trial data used in this article, was supported by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany.
HM, WL and WK received honoraria from Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany.AZ and J Bare employees of Dr. Willmar Schwabe GmbH & Co.
None.

©2015 Mondal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.