Journal of
eISSN: 2373-4396


Research Article Volume 11 Issue 1
1Bioinorganic and Environmental Analytical Chemistry Laboratory, Chemistry Department, Faculty of Basic Science, University of Antofagasta, Chile
2Cardiovascular Surgery Group, Antofagasta Clinic, Chile
Correspondence: Isabel Pizarro Veas, Bioinorganic and Environmental Analytical Chemistry Laboratory, Chemistry Department, Faculty of Basic Science, University of Antofagasta, Antofagasta, Chile
Received: February 23, 2018 | Published: February 28, 2018
Citation: Veas IP, Silva DR, Barrios CS, Arsenic status of cardiovascular tissues from cardiac patients. J Cardiol Curr Res. 2018:11 74-84 . DOI: 10.15406/jccr.2018.11.00377
Arsenic exposure produces non-cancer effects that affect health. Cardiovascular illness due to arsenic exposure has been well documented, but little is known about arsenic in cardiovascular tissues.
We studied the effect of arsenic in cardiovascular tissues from an arsenic exposure heart patient group in Antofagasta, Chile, against a group of non-arsenic exposure patients.
Total arsenic concentrations were measured in 215 pieces of cardiovascular tissue of the arsenic exposure group, and 25 pieces of tissue of the control group. Each patient was asked to complete a questionnaire. Determination of total arsenic was done by HG–AAS, HG–AFS and ICP–MS; speciation analysis was by HPLC–ICP–MS.
Auricle, saphenous veins, mammary arteries, and pooled fat samples from the arsenic exposure group gave concentrations of arsenic within the following ranges: 0.79–13.9; 0.28–13.6; 0.25–10.7; and 0.12–7.70µg/g dry weight; these were greater than that of the control group.
Two-way cluster analysis of the total arsenic concentrations with the demographic variables they allowed to infer that the variables related to medical geology factors are more important that the personal conditional variables with regard to the impact of arsenic on cardiovascular diseases.
Total arsenic concentrations and arsenic speciation revealed that the principal “arsenic target tissues” were the auricles and the mammary arteries.
Knowledge of total arsenic and the prevalence of the trivalent ion (As3+) in the auricle of patients in the arsenic exposure group could contribute to understanding the effect of arsenic on cardiovascular illnesses in countries where arsenic is an important environmental stressor.
Keywords: Arsenic exposure, cardiovascular tissues, arsenic speciation HPLC–ICP–MS, total arsenic concentration clustering; medical geology, demographic conditional variables
Arsenic is present in the coastal (Andean mountain) upper highlands ecosystem in northern Chile due to the volcanic nature of the land, mineralogy of their copper minerals and to the mining exploitation of the same ones,1,2 in this ecosystem the Loa river basin is vitally important for their environmental sustainability because it is the source of water consumption in the main cities and villages of the Antofagasta region. The waters of this dryland river are enriched with arsenic and boron. 3–5
In the last environmental monitoring carried out in September 2005 for our laboratory in eight sampling stations downstream water of the Loa River, the range of concentrations of total arsenic was 0.980–4.17 ppm.
To overcome the effect of arsenic on water quality, arsenic-reduction plants have been in operation since 1972 for the principal cities of Antofagasta. Despite this, the effect of arsenic on human health has been noticeable in relation to cancer effects6‒9 and the incidence of cardiovascular diseases (CVD), which are recognized as typical non-cancer effects due to arsenic exposure.10‒15
Naturally occurring arsenic in the Antofagasta region at North of Chile is due to volcanic activity during the tertiary and quaternary periods in the Andes Mountain, and heavy metals delivery is principally due to copper mining activity,16‒18 which favors the arsenic enrichment of underground and surface waters. The most extensive copper production and contamination with arsenic occur in the area between 17º 30’ and 26º 05’ south latitude and between 67º 00’ west longitude and the Pacific Ocean, usually at altitudes higher than 2,000 m above sea level.1 Small, median and great mining in this area produces over 50% of the copper that is mined in the world. This is the reason why the Antofagasta region is called the “mining capital of Chile” (Figure 1). Mining activity mobilized heavy metals into the environment at rates greatly exceeding those of natural geological processes, disturbing biogeochemical cycles, copper smelting and sulfuric acid plants generate significant emissions of SO2 , arsenic and others heavy metals into the atmosphere, also diffuse pollution may be responsible for the contamination of the environment by toxic pollutants.19
The average concentration of arsenic in the drinking water of Antofagasta was approximately 800µg / L during 1950–1970.6 After installation of arsenic-reduction plants, the arsenic concentration in drinking water of the principal cities of the Antofagasta region decreased to 50µg / L, which it is the maximum level of arsenic in water recommended by the Chilean government. However, there are towns that consume drinkable water with arsenic levels over 50 ppb [6, 20]. Recently, the efficiency of arsenic-removal plants in the Antofagasta region has improved, providing drinking water with arsenic concentrations less than 50µg/L, but higher 10µg/L, which is the maximum tolerable level according to index guidance recommended by the World Health Organization (WHO).21,22
The chronic impact of arsenic in the Antofagasta region produces cancer and non-cancer outcomes, and increases the teratogenic risk of foetal and infant mortality;23 arsenic can easily cross the placentas of humans and animals24 ad also it is a neurological agent.25 Cancer effects8,26 are similar to those described in other countries affected by arsenic in the environment.22,27‒29 According Smith et al.26 and Marshall et al.,8 the impact of arsenic on the population due cancer mortality in the region of Antofagasta in Chile, is greater than reported for any other country to date as a consequence of environmental exposure to carcinogens in a major population, even though major decreases in arsenic exposure occurred more than 25 years earlier due to plants of arsenic depression. Increased lung cancer risk is similar whether arsenic is ingested or inhaled.30
Typical clinical non-cancer effects affecting the health and quality of life of people due to environmental arsenic exposure, such as vascular diseases, abnormal pigmentation, Reynard’s syndrome, acrocyanosis, hyperkeratosis, finger gangrene, tongue ischemia, diabetes, thrombosis, cerebral vascular disease (particularly cerebral strokes), coronary artery occlusions, and other CVDs have been associated to the arsenic chronic exposure.11,14,15,20,27,31‒38 However, other factors of risk like smoking, serum high levels of low-density lipoprotein (LDL cholesterol) and high blood pressure levels have been associated cooperatively with the coronary heart disease (CHD) or cardiovascular atherosclerotic disease in humans. On the other hand, actually it is becoming increasingly evident that low or moderate level exposure to arsenic is widely prevalent35,39,40 for cardiovascular risk. Therefore, it is necessary to obtain more direct evidence about the fate of arsenic in cardiovascular tissues.
The mechanism through which mammals metabolize and detoxify inorganic arsenic involves methylation to methylarsonate and dimethylarsinate of trivalent and pentavalent arsenic, respectively.41‒44 Before methylation occurs, As5+ must be reduced to As3+, forming trivalent methylarsonic acid (MMA) and trivalent dimethylarsinic acid (DMA); these are persistent metabolites and more acutely toxic than the pentavalent forms, and may be more toxic than the trivalent inorganic arsenic ion.45‒47
In healthy humans exposed to trace amounts of arsenic, the highest concentrations are found in tissues rich in sulfhydryl groups (e.g. skin, hair, nails). Little is known about the total concentration of arsenic in the organs of individuals not exposed to trace amounts of arsenic.48 Median arsenic concentrations (in dry weight) in the organs of healthy people range from 0.012µg/g in the brain to 0.46µg/g in hair, while concentrations in the organs of healthy people from Japan (who generally consume relatively high amounts of seafood) range from 0.02µg/g wet weight to 0.89µg/g in nails.49 In fatal cases of arsenic poisoning, the concentrations of arsenic in tissues changed and exceeded the above values, showing widespread distribution of arsenic in all organs, the highest levels (in decreasing order) were observed in liver, kidney, brain, lungs,
heart, pancreas, spleen, and muscles.48 However, comparative studies of trace elements and heavy metals in cardiovascular tissues are yet difficult due to the lack of “normal" values and arsenic speciation in human tissues is scarce.48‒50 Most studies of arsenic speciation have focused on water, plants and animal tissues, and human fluids (e.g. urine) and tissues such as hair and nails.51
In this work we detail the distribution of the total arsenic concentration in cardiovascular tissues, obtained by heart surgery of a group of heart patients subjected to chronic arsenic exposure of the Antofagasta region, and in the same cardiovascular tissues from a control group of patients subjected to the same surgery type of regions of Chile without arsenic exposure coming from drinking water. The cardiovascular tissues were auricle (AU), mammary artery (MAM), saphenous vein (SAP) and the pooled fat sample from these cardiovascular tissues (FAT). Also, we report the results of arsenic speciation in the cardiovascular tissues of four heart patients, i.e. the distribution of arsenic species (As5+, As3+ and their metabolites) in three patients from the arsenic exposure group and one from the control group. Knowledge of the total arsenic levels and the distribution of the main arsenic species present in these tissues could aid understanding of the long-term effect of arsenic on vascular and cardiovascular diseases.
For some geological areas of the planet, the adverse effects of arsenic exposure on human beings are a truth public health problem,7,22,52,53 that which also involve to the Medical Geology. Medical Geology is the science that deals with the impact of geologic materials and processes on animal and human health.54-56
This work was carried out according to the Helsinki II declaration, with the consent and authorization of the Antofagasta Clinic. It was approved by the Committee of Ethics of the University of Antofagasta (CBIC REV 1/2005).
Sample populations
The samples of cardiovascular tissues of the group in study were taken up from 215 patients subjected to heart surgery in the Antofagasta clinical, which have lived in the region of Antofagasta for at least five years. Control group samples were obtained from 25 heart patients operated in the Hospital of the Catholic University in Santiago; they came from Chilean regions without drinking water arsenic exposure. In the Antofagasta Clinic, pieces of auricle (AU), mammary artery (MAM), and saphenous vein (SAP) were collected for the arsenic exposure group between the years 1995 and 2000; the fatty residuals (FAT) of each sample of cardiovascular tissue were joined, homogenized, and considered as pooled samples. The patients had been subjected to heart surgery due to arterial thrombosis and to each one of them was consulted a voluntary questionnaire (Table 1).
As exposure group |
As control group |
|
Cardiac patients |
215 |
25 |
Age |
||
Mean |
57 |
60 |
Median |
56 |
59 |
Min–Max |
33–78 |
38–78 |
Variables influenced by |
As exposure group, % |
As control group, % |
medical geology factors |
||
BA |
72.1 |
- |
NBA |
21.9 |
- |
CN |
29.3 |
- |
RS |
25.1 |
- |
OC |
40 |
- |
WA |
47.9 |
- |
WCh |
22.8 |
- |
OW |
12.1 |
- |
NMW |
13.5 |
- |
Conditional variables |
As exposure group, % |
As control group, % |
S |
64.7 |
60 |
NS |
31.2 |
40 |
W |
46 |
56 |
NW |
48.4 |
44 |
L |
18.6 |
- |
NL |
77.7 |
100 |
D |
73.5 |
32 |
WD |
20.9 |
68 |
F |
14 |
16 |
M |
81.4 |
84 |
Table 1 Demographic questionnaire data (a) for the arsenic exposure and control cardiac patients groups
BA, patients born in the Antofagasta region and who have always resided in this region; NBA, patients not born in the Antofagasta region, but who have lived there at least 5 years; CN, inhabitants patients of the centre–north zone of Antofagasta city; RS, patients living in the south zone of Antofagasta city; OC, patients of other cities of the Antofagasta region; WA, patients that worked in Antofagasta city; WCh, patients that worked in the mines of Chuquicamata; OW, patients that worked in other copper mining locations, thermoelectric power plants and saltpeter mining; NMW, patients that worked in different roles to mining and power generation; S, patients who smoked; NS, patients who did not smoke; W, patients who consumed wine moderately; NW, patients who did not consume wine; L, patients with leucomelanosis; NL, patients without leucomelanosis; D, dislipidemic patients; WD, patients without dislipidemia; M, male patients; F, female patients.
Manipulations and procedures for sample preparation were made in a “clean laboratory” inside a laminar flow hood (Labconco, Purifier Class II, USA) using inert devices (e.g. plastic and titanium knives, agate grinding mortar) and scalpels, scissors and forceps of surgical stainless steel. After removing the titanium clasp from the tissues, the samples were rinsed with deionized water. Samples were stored at –20ºC before use. Dry/wet weight factors were obtained according to the UNEP protocol for biological tissues.57
Mineralization of sample tissues for determination of total arsenic contents
To assure the complete destruction of the fatty tissues, the sample mineralization was carried out in Teflon reactor bombs heated in a home made refractory oven with internal temperature sensor and external control, according a two step mineralization procedure.58 0.5–1.0 g of sample was attacked in the Teflon reactor bomb with 10 mL of concentrated HNO3, 2 mL of concentrated HClO4 and 2 mL of 2% m/v Na2S2O8. Samples were pre-digested overnight at room temperature and the reactor bombs heated to 150ºC for 2 hours in the refractory oven. After cooling, 0.5 mL of concentrated H2SO4 was added, and the digested sample heated in an aluminium heating plate from ambient until 300ºC in a timing of 85 min at semi-refluxing into a 50 mL glass Erlenmeyer flask, the final volume was 2 mL approximately. The digested sample was diluted to 10 or 25 mL with 0.5 M HCl and the total arsenic was determined by HG–AAS. Deionized water was used for making up to the chosen volumes when the arsenic measurements were done by ICP–MS.
Materials, reagents and standards
HNO3 and H2SO4 were Suprapur grade (Merck); HClO4 and Na2S2O8 were INSTRA grade (J. T. Baker). NaBH4, NaOH, (NH4) H2PO4, H3PO4, and NH3 were Merck p.a. Each stock solution of arsenic species compounds containing 1.000 g/L of As were prepared by dissolving the respective amount of the following reagent in water: inorganic As(III) and As(V) standards from sodium arsenite and sodium arsenate (Sigma Aldrich, St Quintin, Fallavier, France); DMA (Merck), MMA (Chem Service, USA); arsenobetaine (AsB) and arsenocholine (AsC) (Tri Chemical Laboratory Inc. Japan); stock solutions were kept at 4ºC in the dark. Experimental solutions were prepared daily and diluted with water to the final concentration. The standard reference materials (SRMs) DORM-1 (dogfish muscle), DORM-2 (dogfish muscle), TORT-1 (lobster hepatopancreas) and LUTS-1 (non-defatted lobster hepatopancreas) from the National Research Council Canada (NRCC), were used to validate the total arsenic determinations.
The methanol–water solutions were prepared with deionized water (Milli-Q Ultrapure water systems, Millipore, USA) and HPLC-grade methanol (Merck). Sonication of samples was done in a focused ultrasonic bath (Bandelin Sonopuls HD-2200, Fungilab Corp., USA). Solvent evaporation of extracts was carried out in a rotavapor Univapo100H-Unijet II (UNIEQUIP, USA).
Determination of total arsenic
After tissue mineralization, total arsenic contents were measured by hydride generation atomic absorption spectrometry (HG–AAS). Otherwise, total arsenic concentrations in methanol–water extracts and in their digested residues were measured by inductively coupled plasma mass spectrometry (ICP–MS). Multiple standard addition calibration was used for HG–AAS. For ICP–MS measurements 75As(V) standard solution was used for calibration and control with 72Ge (10µg/L) as internal standard.
Instrumentation and chromatographic materials
HG–AAS measurements for total arsenic concentrations were done on GBC 909 PBT equipment coupled with a GBC HG-3000 hydride generator coupled with an electrothermal mantle GBC EHG-3000 (Australia) in the Chilean samples. One arsenic hollow cathode-boosted discharge lamp (BDL) from Photron (Australia) was used. An ICP–MS HP-4500 (Yokogawa Analytical System, Tokyo, Japan) was used for the detection of arsenic after separation of arsenic species using HPLC. A Babington glass nebulizer coupled to a Scott double-pass spray chamber; single ion monitoring at m/z of 75As was used to collect the data. Signal quantification was done in the peak area mode.
Fractionation of arsenic in cardiovascular tissues
Cardiovascular tissues of three patients of the arsenic exposure group and one of the control groups were treated for the arsenic speciation study according Shibata and Morita.59,60 Hence, 0.5–1.0 g of cardiovascular tissues previously dried at 60ºC was placed in plastic centrifuge tubes with 10 mL of 1:1 methanol–water (v/v). They were shaken mechanically for 3 hours; the tubes were maintained at 55ºC for 10 hours and finally left in an ultrasonic focalized bath for 5 min. Samples treated this way was centrifuged for 15 min at 6,000 rpm and the extract removed. Insoluble residue was re-extracted using 5 mL of the same methanol–water mixtures under the same conditions, and the solid residual was extracted with 9:1 methanol–water v/v solution using the procedure described above. The 1:1 and 9:1 methanol–water extracts were separately dried by rotary evaporation at 40ºC with a flow of ultra-pure N2, and then separately dissolved in an appropriate volume of deionized microfiltered water (0.45µm) and frozen to –20 ºC before analysis. Triplicates extracts were prepared from each one cardiovascular tissue sample from the four patients in which the arsenic speciation was studied, and each one of the reference material LUTS-1, DORM-1, DORM – 2 and TORT – 1 for quality control measurements.
HPLC–ICP–MS for measurement of arsenic speciation
A PRP–X100 analytical coupled with a guard anion-exchange column (Hamilton, Reindeer, Nevada, USA) were used for HPLC–ICP–MS hyphenated analysis. For the HPLC chromatographic separations, 100µL of samples were introduced through a 0.45-µm nylon syringe filter into the injection valve Rheodyne 9125 (USA) and then pumped into the HPLC system (Milton Roy LDC Division, FL., USA); air was removed from the buffers by argon degassing for 15 min and the buffer filtered before injection. The column effluent was directly introduced into the nebulizer rod of the ICP–MS equipment by a polytetrafluoroethylene capillary tube of dimension 250 mm × 0.5 mm (id). The ion intensity at m/z of 75As was monitored, but an important interference during ICP–MS analysis is the possible formation of 40Ar35Cl, however chloride concentrations in the analyzed fractions were low and the eventual interference was corrected by the correction factor introduced in the operational software of the instrument. Peaks were integrated using the software ICP–MS Lab and Captures Grams/32 (Galactic Industries Salem NY, USA). An injection of 100µL of 5.0 ng/mL of 75As(V) was made, along with the internal standard (72Ge) before each chromatographic run in order to correct drift in the ICP–MS response. Table 2 shows the optimized instrumental parameters for the application of the HG–AAS, ICP–MS and HPLC techniques.
HG–AAS |
|
NaBH4 concentration |
3% |
HCl concentration |
3 M |
NaBH4 and HCl flow rate |
2.0 mL/min |
Sample flow rate Reaction coil length |
0.8 mL/min |
Measurement mode |
25 cm |
High peak |
|
ICP–MS |
|
RF Power |
|
Forward: 1350 W |
|
Reflected: 2.2 W |
|
Ar flow rate |
|
Coolant: 14 L/min |
|
Nebulizer: 1.0 L/min |
|
Auxiliary: 0.9 L/min |
|
Peak area, 75As |
|
Measurement mode (integration peaks per points) |
|
3 replicates |
|
HPLC column |
|
Mobile phase |
Anionic PRP X-100 |
(NH4)2HPO4, pH 6.0 |
|
Gradient mode (%) |
|
Solution A: 5 mm; Solution B: 25 mm |
|
0-15 min (A: 100-0 and B: 0-100) |
|
15-25 min (A: 0-100 and B: 100-0) |
|
Flow rate |
Conditioning: 25-30 min (A: 100 and B: 0) |
Sample injection |
1.5 mL/min |
100 µL |
|
Table 2 Instrumental conditions applied in the analytical measurements of arsenic
Analytical validation, quality control and traceability of arsenic measurements
Table 3 summarizes the analytic validation data to prove the suitability and efficiency of the described techniques for the determination of arsenic in cardiovascular tissues; i.e. total arsenic, total extracted arsenic with methanol–water 1:1 and 9:1 and the arsenic recovery experiments from the standard reference materials spiked with arsenic species frequently found in biological tissues, such as primary standard of As3+ and As5+ of sodium arsenite and sodium arsenate (Sigma Aldrich, St Quintin, Fallavier, France); DMA (Merck); monometilarsonic acid (MMA) (Chem Service, USA); AsB and arsenocholine (AsC) (Tri Chemical Laboratory Inc. Japan). The SRM’s used in these quality control approaches were DORM-1, DORM-2, TORT-1 and LUTS-1 (National Research Council, Division of Chemistry, Ottawa, Canada). Detection limits were calculated in accordance with IUPAC criteria.61 The results of the measured values of arsenic were accepted if the variation coefficient and the relative error tests were less to 15%, respectively.
Before being applied to the cardiovascular tissue samples, the arsenic speciation protocol was applied to standard reference material samples (Table 3). These results are very similar to obtained in other reports.62‒65
|
Total As by HGAAS |
As found in MeOH – Agua 1:1 extract by HGAAS (a) |
As found in MeOH – Agua 1:1 extract by HGAFS (a) |
As found in MeOH – Agua 1:1 extract |
|||
N |
4 |
5 |
5 |
5 |
|||
SRM |
DORM-1 |
TORT-1 |
TORT-1 |
TORT-1 |
|||
Average found concentration |
17.2 |
21.2 (22.9) |
21.5 (23.1) |
21.7 (23.4) |
|||
Certified concentration |
17,7 |
24.6 |
24.6 |
24.6 |
|||
RE (%) |
- 3.1 |
6.9 |
6.1 |
4.9 |
|||
RSD (± %) |
7.9 |
7.7 |
8.3 |
5.8 |
|||
CL (ng/mL) |
0.69 |
0.22 |
0.24 |
0.09 |
|||
Spiked arsenic species measurements in Me-H2O 1:1 SRM extracts by HPLC – ICPMS |
|||||||
SRM |
N |
As Species |
Species found(µg/g) |
RSD(± %) |
Species spiking |
Species recovery (%) |
CL |
TORT – 1 |
3 |
As3+ |
Nd |
- |
50 ng/mL |
98.6 |
0.02 |
LUTS – 1 |
3 |
As3+ |
Nd |
- |
50 ng/mL |
97.2 |
0.02 |
DORM – 2 |
3 |
As3+ |
Nd |
- |
50 ng/mL |
96.6 |
0.02 |
Table 3 Quality control and traceability of the total As concentrations, MeOH–H2O 1:1 fractionated arsenic concentrations and speciated concentrations of arsenic using standard reference materials (SRM).
(A) Mean total concentrations of Arsenic found in the standard reference materials are between parentheses
Statistics
Statistical analyses were carried out using the statistical package STATISTICA 6.1 (StatSoft, Tulsa, OK); p < 0.05 was considered statistically significant.
Demographic questionnaire
According to the definition of the science of human exposure,66 we have assumed that in an ecosystem subject to environmental stressors coming from geological sources and anthropogenic activities, it is possible to define a group of possible factors or conditions that could be considered candidate-predictors or variables that could describe the effects on humans of environmental stressors like arsenic exposure in the Antofagasta region, Chile. From this viewpoint, it is possible to discriminate subjects influenced by medical geology factors (e.g. metal- and metalloid-enriched geographical areas) and conditional variables associated with risky lifestyles such as to smoke and drinking wine, sanguinity indexes, or gender that could predispose to cardiovascular disease after exposure to arsenic. Medical geology offers the opportunity to identify and characterize links between the natural environment and human health.54‒56 Table 1 shows the results of the demographic questionnaire consulted to patients of the groups chronically exposed to arsenic for consumption of drinkable water and control.
Statistical treatment
Table 4 shows the statistical results of the total arsenic concentration in the cardiovascular tissues of the Antofagasta region cardiac patients group and the control cardiac patients group. Application of the Shapiro–Wilkinson test shows that a normal (p=0.05) distribution is not followed in both groups of cardiovascular tissues under study, but rather the distribution of the total arsenic data biased to be lognormal, this model has been proposed for representing the frequency distribution of the concentrations of trace metals found in human tissue.67 Application of the student t-test for independent groups (p=0.05) demonstrated that the arsenic concentrations in AU and MAM tissues were significantly different between both groups of patients, but not for SAP and FAT tissues. The highest arsenic concentrations in the cardiovascular tissues of the arsenic exposure and control cardiac patient groups were found in AU and SAP tissues, respectively, but the arsenic concentrations in the SAP tissue of both groups of heart patients were not significantly different. It can be inferred then, that the concentrations of total arsenic in the tissues of the control group were higher than that waited, that which can be mainly consequence of the impact for inhaled arsenic.
Cardiovascular tissues |
N |
Mean |
SD (±) |
Min–Max |
Median |
Auricle, As exposure group |
215 |
4.85 |
3.85 |
0.40 –29.4 |
3.79 |
Auricle, control group |
22 |
2.56 |
2.51 |
0.52 – 9.75 |
1.99 |
Mammary, As exposure group |
206 |
1.52 |
1.85 |
0.13 – 13.1 |
0.9 |
Mammary, control group |
25 |
0.56 |
0.27 |
0.20 – 1.35 |
0.51 |
Saphenous, As exposure group |
199 |
2.97 |
2.95 |
0.11 – 23.8 |
2.13 |
Saphenous, control group |
25 |
3.79 |
3.89 |
0.38 – 15.6 |
1.99 |
Fatty CV tissue, As exposure group |
211 |
1.27 |
1.64 |
0.076 – 14.3 |
0.78 |
Fatty CV tissue, control group |
25 |
0.72 |
0.54 |
0.24 – 2.60 |
0.51 |
Table 4 Statistical parameters for the total arsenic concentrations in cardiovascular tissues of the arsenic exposure group and control groups. Concentrations in µg/g dry weight.
The total concentrations of arsenic in the cardiovascular tissues of patients of the arsenic exposure group were associated to the demographic questionnaire data, for which the variables described above were considered (Table 1). The total arsenic concentrations in the cardiovascular tissues of control group patients were associated with conditional variables, i.e. patients who smoked (Sc), patients who did not smoke (NSC); patients who consumed moderately wine (Wc), patients who did not consume wine (NWc); patients with dislipidemia (Dc), patients without dislipidemia (WDc); male patients (M) and female patients (F). When the arsenic concentrations are associated with the variables influenced by geomedical factors, the application of the student test to the arsenic concentrations in auricle (AU), mammary artery (MAM) and saphenous vein (SAP) of the exposed group to arsenic, demonstrated that significant differences do not exist among the arsenic concentrations inside oneself type of cardiovascular tissue. It can be inferred that the arsenic concentrations on the cardiovascular tissues of cardiac patients of the Antofagasta region are independent of where they live and work in the Antofagasta region. There were significant differences in the arsenic concentrations in the pooled fat cardiovascular samples for several pairs of variables related to medical geology factors: RS vs BA, CN, OC, WCh, NMW, and WA vs NMW. RS correspond to patients with residence at the south zone of Antofagasta city, which is near to copper smelting facilities.
The associative linking among arsenic concentrations in the cardiovascular tissues of the arsenic exposure group and control group with conditional variables (Table 1), allowed by means the student t-test (p=0.05) to identify statistical difference between the arsenic concentrations in the cardiovascular tissue of both groups of cardiac patients. For AU, the differences were significant for the variables NS, NW, NL, D, and M; in MAM, the differences of arsenic concentration between both groups were significant for W, NL, D and M; in SAP, the differences in arsenic concentration between both groups were significant alone for W and M. For FAT, they were not significant differences between the arsenic concentrations from the arsenic exposure group and control group. In this way, the box plots of the Figure 2 show the arsenic concentrations profiles in cardiovascular tissues translated according to the conditional variables of both groups. The plateau of the medians of the arsenic concentrations in AU, MAM and FAT of the arsenic exposure group is approximately twofold major than the arsenic concentrations in the same tissues of the control group. In SAP of the control group, some cases presented median values for arsenic concentrations higher than that of the corresponding cases of the arsenic exposure group. The above results permit infer that the influence of the medical geology factors are more determinative than conditional variables for the arsenic enrichment in cardiovascular tissues of humans; for another part, heart auricle (AU) and mammary artery (MAM) could be good biomarker for arsenic exposure. Arterial vessels have already been proposed as biomarkers for the exposure to other heavy metals.68
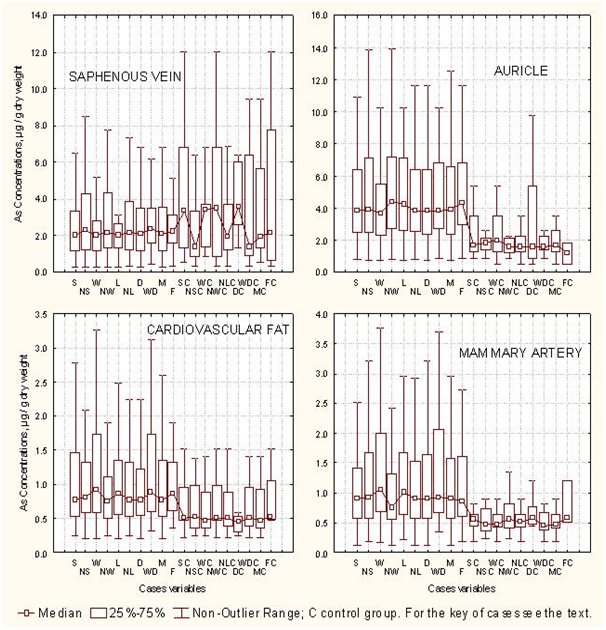
Statistical multivariate treatment
From this point of view, we were interested in carrying out a purely phenomenological interpretation, without “a priori” suppositions regarding the distribution of arsenic concentrations in the cardiovascular tissues in the two groups of heart patients in study. Cluster analysis is an exploratory data analysis procedure, hence it is usually applied to data sets for which there is no a priori knowledge concerning the class membership of the samples, whose basic objective is to discover sample or variable grouping within data.69 There are two important issues, the way of measuring the distance between samples (metrics) and the way of measuring the distance between samples and cluster (linkage rule).70 In the present work, we applied cluster analysis to measure the distance among the variables (metrics) considering the method of Ward with the approach of 1 – Pearson r to measure the distance between the variables and the cluster (linkage rule). On the other hand, the two – way joining plotting technique was also applied.
The dendrogram of the arsenic concentrations in the cardiovascular tissues of the arsenic exposure and control groups. Figure 3A shows clustering of the tissues that follow two principal patterns, i.e. the arsenic exposure group and the control group, respectively. Two-way clustering was applied to explore the bidimensional multivariate relationship of arsenic concentrations in the cardiovascular tissues linked with demographic variables (Table 1), but as the distribution of the arsenic concentrations in the tissues turned out to be lognormal, the data were regrouped considering as more representative value of each one from the variables to the median of the arsenic concentrations.
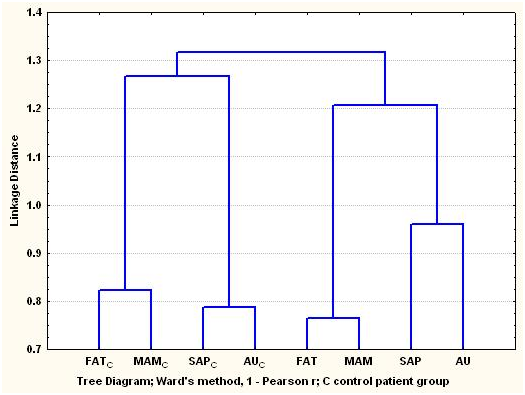
Figure 3a Dendrogram of the As concentrations in cardiovascular tissues of arsenic exposure cardiac patients group from the Antofagasta region in northern Chile, and the control cardiac patients group coming from regions in central and southern Chile.
Dendrogram of the As concentrations in cardiovascular tissues of arsenic exposure cardiac patients group from the Antofagasta region in northern Chile, and the control cardiac patients group coming from regions in central and southern Chile.
Figures 3B & 3C show the associative distribution with the demographic variables, i.e. the two-way clustering regarding the conditional variables for both cardiac patients groups and the clustering of the arsenic exposure group cardiovascular tissues regarding the arsenic concentrations considering to all demographic variables. In the first case, we can infer that the arsenic appears transversally associated to AU tissue independently of conditional variables. In the second case, when all the variables were considered, the cardiovascular tissue preferred by arsenic was also the auricle (AU), but it is possible to observe that the most important associations show up among the arsenic concentrations in auricle (AU) and the variables influenced by medical geology factors. These include patients with residence in other cities of the Antofagasta region (OC); patients who work in Antofagasta city (WA); patients who work in the copper mines of Chuquicamata (WCh), and for some conditional variables, such as patients who not consume wine (NW); patients with leucomelanosis (L) and female patients (F). In the control group they did not show up patient with leucomelanosis, but not all the patients of the exposed group to arsenic presented corporal stigmata, for what is possible that this condition be induced by medical geology factors.

These data allow us to conclude that the cardiovascular tissues, in particular the auricle (AU) and mammary artery (MAM) tissues, are “good biomarkers tissues” of the risk to cardiovascular health due exposure to arsenic. This way, it seems not to be apparent that more traverse medical geological factors than those considered in this work, would be fundamentally determining the risk for the cardiovascular health in human beings exposed to arsenic in the region of Antofagasta – Chile. These factors include exposure to essential and non essential trace elements from the source water for human consumption,6,26 consumption of agricultural products cultivated in arsenic-enriched soils watered with arsenic-enriched underground waters,17 inhalation of heavy metals from the atmosphere due to industrial activity, climatic factors, and the consumption of sea foods.19,30,71‒73 The current concentrations of total arsenic in the drinkable water of the city of Antofagasta after being treated in arsenic-reduction plants are between 15.0 ppb to 30.0 ppb; this satisfies the Chilean guide of 50 ppb, but not the WHO guide of 10 ppb.
Arsenic species found in cardiovascular tissues
Table 5 shows the results of the arsenic fractionation in methanol–water mixtures59 and the IC–HPLC–MS chromatographic speciation of the cardiovascular tissues of three heart arterial thrombosis patients of 37, 44 and 53 years old, who had lived in the Antofagasta region. These results show that most of the arsenic in the tissues examined was extracted by the 1:1 methanol–water-extracting solution; this has also been observed in other biological tissues.60,65 Under these conditions, the arsenic species contained in these cells could be extracted and the arsenic speciation investigated, so we can assume that at least the 1:1 methanol–water extract contains the arsenic species of the cytosol solution.
The arsenic speciation protocol described in this work was applied separately to the AU, MAM, SAP and FAT tissues of each of the three cardiac patients from the arsenic exposure group of the Antofagasta region, and to one cardiac patient of the control group who had lived in the Valparaíso region all of his life. The arsenic speciation results are shown in Table 5. Figures 4A & 4B show the HPLC–ICP – MS arsenic chromatographic speciation profiles of auricle (AU) and mammary artery (MAM) cytosols of the cardiac patient with the highest arsenic enrichment. The chromatograms of the arsenic speciation of this same cardiovascular tissues of the patient from Valparaíso region were similar to the previous ones (not shown), but the concentrations of the arsenic species were lower.
The arsenic speciation results in the cardiovascular tissues studied in this work shows that only arsenite (As3+) and arsenate (As5+) species were found in the citosol of the auricle (AU) tissue (Figure 4A). Both inorganic arsenic species were confirmed by spiking the standard solutions of the species; arsenite was also confirmed after being separated by means of cationic chromatography using the cationic column Hamilton PRP – X 200 (chromatograms not shown), in which As3+ does not overlap with other arsenic species. Arsenite was also the predominant species in the citosol of the saphenous vein (SAP), which suggests that the arsenite could be the main arsenic species in the cytosol of this tissue, but a small amount of DMA species were also found (chromatogram not shown). In the citosol of the mammary artery (MAM), (Figure 4B), the major arsenic species were arsenate and AsB, DMA and MMA were only minor species; arsenite was not found. In the citosol of the pooled fat of the cardiovascular tissues (FAT), in this case AsB was the predominant species, followed by arsenate and DMA (chromatogram not shown). The arsenic species stability was monitored during sample preparation and storage.65
MMA and DMA are the main products of the cellular biomethylation through the conjugated effects of S–adenosylmethionine and the methyltransferase enzyme, but presently work was not discriminated against between As(III) and As(V) methylated species, the spiking additions were made with As(V)-methylated arsenicals. As(V) is the main arsenic species in surface natural waters, therefore the biotransformation of As(V) to As(III) must be a main mechanism in the organisms. In general, MMA and DMA species have been considered important in arsenic detoxification mechanisms, so it was surprising that in this work DMA and MMA species were not detected in the citosol of auricle (AU) and the concentration of DMA was very low in the citosol of saphenous vein (SAP) and mammary artery (MAM) (Table 5). Similar results were found in heart tissues of chickens.74 AsB is believed to have a very low toxicity and has been found in human serum,21 but the presence of Arsenobetaine was unexpected in this work; this organarsenical species enters human beings principally from marine foods and from agricultural products coming from polluted soils and/or watered with arsenic-enriched waters.17,21
|
Auricle |
Mammary artery |
Saphenous vein |
Fat tissue |
Total As (µg/g) |
6.9±2.1 |
0.9±0.2 |
4.96±1.2 |
0.8±0.2 |
Fractionation and chromatographic arsenic speciation (a) |
||||
MeOH–H2O (1:1) |
70.9±3.1 |
60. ±2.7 |
56.2±2.9 |
67.4±2.9 |
MeOH – H2O (9:1) |
19.3±2.0 |
26.2±2.5 |
29.7±2.9 |
30.3±2.2 |
Residual As |
9.1±1.8 |
12.0±1.1 |
12.2±1.3 |
1.9±0.7 |
Table 5 Fractionation and chromatographic speciation (HPLC – ICP – MS) of arsenic in cardiovascular tissues of arsenic exposure patient group from the region of Antofagasta at North of Chile.
Mean percentages (wet weight basis) of the tissues of three cardiac patients regarding their average total arsenic concentration; Nd, not detected.
In vivo methylation has long been proposed as an arsenic detoxification pathway for inorganic arsenic,44,75 but it has been shown that As(III)–MMA and As(III)–DMA, two intermediates in arsenate methylation, are more toxic to cells than As3+ and As5+ ions.76‒78 Arsenic is primarily metabolized in the liver and cardiovascular tissue probably does not efficiently metabolize arsenic. The inefficiency of methylation mechanisms could be due to the lack of methylating agents in cardiovascular tissues.
We report, for the first time, the total concentrations of arsenic in cardiovascular tissues, obtained via heart surgery, from a group of arsenic-exposure cardiac patients who have lived in Antofagasta, an area at the North of Chile with exposure to arsenic in drinking water, in relation to a control group of cardiac patients who have lived in regions of Central and South of Chile. Arsenic speciation status was also investigated in the cardiovascular tissue citosol of three patients subjected to heart surgery from the arsenic exposure group, and in one patient from the control group. Knowledge of total arsenic level concentrations and the speciated distribution of arsenic in the citosol of cardiovascular tissues, in particular, the prevalence of As3+ in the auricle of the arsenic exposure group, will aid the understanding the long-term effects of arsenic on cardiovascular and vascular illnesses. The results obtained in this work supplemented with recent epidemiologic evidence;14,79 allow us to conclude that the cardiovascular tissues are good biomarker tissues of the risk to cardiovascular health due to arsenic exposure. In particular, it was demonstrated that the auricle is an “As3+ target tissue”, which is considered one the most toxic arsenic species.
Otherwise, linking of the total concentrations of arsenic with conditional variables and variables related to medical geology factors considered in this work, allowed us to infer that the latter are more important for discriminating the cardiovascular risk for arsenic exposure in the Antofagasta region.
This research was supported by resources generated by the Bioinorganic and Environmental Analytical Chemistry Laboratory of the University of Antofagasta, through the University of Antofagasta Technical Attendance, and by a grant under the EU’s RTD programme and the SEAS project. The authors have no competing financial interest.

©2018 Veas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.