Journal of
eISSN: 2373-4396


Case Report Volume 11 Issue 1
1Texas Health Presbyterian Hospital of Dallas, USA
2Presbyterian Heart and Vascular Group, Texas Health Physicians Group, USA
3Infectious Care, USA
4Dallas Nephrology Associates, USA
5Department of Radiology, Texas Health Presbyterian Hospital of Dallas, USA
Correspondence: Andrew Davis Assaf, PGY-2 Internal Medicine Resident Physician, Texas Health Presbyterian Dallas, 8130 Park Ln Apt 1012, USA, Tel 281 743 4849
Received: December 30, 2017 | Published: January 17, 2018
Citation: Assaf A, Cheirif J, Liddell A, Wall B, Bowman R et al. (2018) Myopericarditis in a Young Turkish Woman with Heterozygous Familial Mediterranean Fever. J Cardiol Curr Res 11(1): 00366. DOI: 10.15406/jccr.2018.11.00366
Familial Mediterranean Fever (FMF) has many different clinical presentations, the most common of which is fever with serositis. Myopericarditis may occur in patients with FMF. We present the case of a young Turkish female who had an extensive workup for fever, abdominal pain, and mesenteric lymphadenopathy and who later developed myopericarditis. Subsequent genetic testing revealed that the patient was heterozygous for the E148Q mutation in the MEFV gene. The patient was treated with an NSAID initially and was then transitioned to colchicine, with a significant improvement in the frequency of abdominal pain attacks and episodes of high grade fevers and without recurrence of myopericarditis.
Keywords: familial Mediterranean fever (FMF), myopericarditis, mesenteric lymphadenopathy
Myopericarditis is usually a self-limiting disease, most commonly caused by a viral infection. The most frequent viral etiologies are coxsackie B virus, adenovirus, herpes viruses, hepatitis C virus, parvoviruses and echovirus, among others.1,2 An underlying autoinflammatory disorder, though uncommon, may also cause myopericarditis. We present a patient with fever, mesenteric lymphadenopathy and abdominal pain who later developed myopericarditis and was subsequently found to be heterozygous for the E148Q mutation in the MEFV gene
A 16-year-old girl was referred to the Infectious Diseases clinic at our facility in Dallas, Texas on 1/9/2015 for abdominal pain and fever. The patient had no pertinent past medical history, nor was she taking any medications prior to the onset of her illness. Her symptoms began 6 months earlier when she was working in Missouri as a counselor at a camp for disabled children. At that time, she developed fatigue, fever, sinus pain and nasal drainage. She was treated with amoxicillin/clavulanate for a clinical diagnosis of sinusitis. Most of her symptoms resolved but she still had fatigue. In the interim, she developed intermittent bilateral hip, knee, and ankle pain in addition to intermittent right lower quadrant abdominal pain. She saw a physician in November 2014 and had routine laboratory studies which were unrevealing. She was prescribed another course of amoxicillin/clavulanate, but she continued to have fatigue and later developed high grade fevers without a particular pattern. CT scan of the abdomen revealed mesenteric lymphadenopathy and possible terminal ileitis. The erythrocyte sedimentation rate and C-reactive protein level were reportedly within normal limits. She underwent an upper and lower endoscopy which were normal. An elective appendectomy revealed only chronic inflammation, without acute appendicitis or granulomas.
The patient developed non-radiating, mid-sternal chest pain that worsened on inspiration with associated shortness of breath on 1/20/2015 and presented to our emergency department. On arrival, her blood pressure was 112/56 mmHg, heart rate was regular at 118 beats per minute, temperature was 97o F and respiratory rate was 16 breaths per minute.
Her pulse oximetry revealed an oxygen saturation of 100 % on room air. Body mass index was 19 kg/m2. She was a well-developed and well-nourished young female in no acute distress. Her oropharynx was normal. Her cardiac examination revealed sinus tachycardia, normal S1 and S2 with no pericardial knock, friction rub, murmurs, or gallops. A joint examination was normal. There was no rash. Pulmonary and abdominal examinations were also unremarkable.
ECG revealed sinus tachycardia and right axis deviation without ST-T changes (Figure 1.). Laboratory studies, summarized in Table 1, revealed a mild normocytic, normochromic anemia and a considerably elevated serum troponin I level. Chest radiography (Figure 2) revealed a non-specific mild left perihilar/lingular area of atelectasis or infiltrate. Given her history of fever, the patient underwent technetium labeled white blood cell (WBC) scanning, prior to the onset of her chest pain, which revealed intense labeled WBC uptake at the left ventricular myocardium consistent with myopericarditis (Figure 3). A transthoracic echocardiogram (Figure 4) revealed normal right and left ventricular (left ventricular ejection fraction of 55-60 %) and a small pericardial effusion.
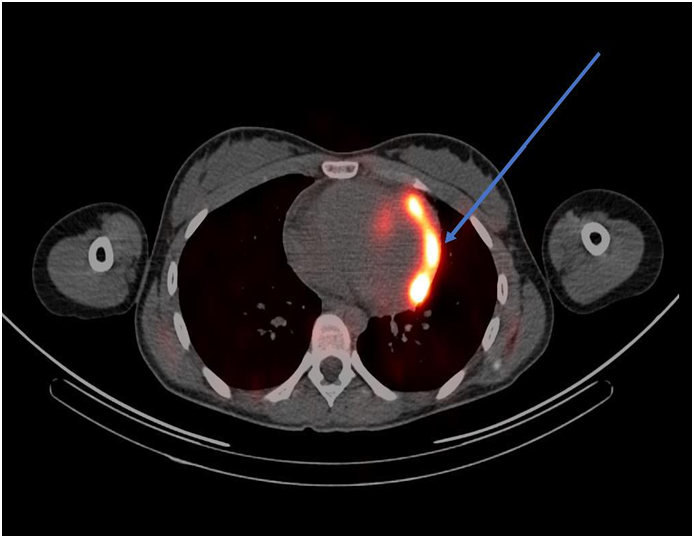
Figure 3 Technetium labeled WBC scan (arrow indicating significant uptake in the left ventricular myocardium).
|
Value |
Reference Interval |
|
|
WBC |
9 x 103/µL |
4 – 10 x 103/µL |
|
RBC |
4.08 x 106/µL¯ |
4.1 – 5.1 x 106/µL |
|
HGB |
11.6 g/dl ¯ |
12– 16 g/dl |
|
HCT |
34.8 ¯ |
36– 46 % |
|
MCV |
85.3 fL |
79– 98 fL |
|
Platelet Count |
165 x 103/µL |
130 – 400 x 103/µL |
|
D-Dimer |
0.2 µg/ml |
≤ 0.5 µg/ml |
|
BUN |
14 mg/dl |
10-20 mg/dl |
|
Creatinine |
0.71 mg/dl |
0.57 – 1.11 mg/dl |
|
Troponin I |
1.125 ng/ml |
≤ 0.036 ng/ml |
|
TSH |
2.657 µIU/ml |
0.34 – 4.792 µIU/ml |
Table 1 Laboratory findings
The patient was treated with ibuprofen 400 mg BID and colchicine 0.6 mg daily and her symptoms improved. However, her fatigue persisted, and she underwent a laparoscopic excisional biopsy of a mesenteric lymph node to exclude lymphoma or another neoplasm. Histopathology of the excised lymph node revealed a preserved lymph node architecture without granulomas or neoplastic cells, but with paracortical hyperplasia. Given the history of myopericarditis, reactive mesenteric lymphadenopathy, fever and chronic fatigue in a young female originally from Turkey, evaluation for FMF was considered. Subsequent genetic testing revealed that the patient was heterozygous for the E148Q mutation in the MEFV gene. Her colchicine dose was increased to 0.6 mg twice daily. She had no further recurrence of myopericarditis and the frequency and severity of abdominal pain and high grade fevers were significantly reduced after initiating therapy with colchicine. Serum electrolyte levels, fasting blood glucose level, liver chemistry tests, serum albumin, globulin and total bilirubin levels were all within the respective reference intervals (not shown).
Familial Mediterranean fever (FMF) is an autosomal recessive, hereditary, autoinflammatory disorder caused by mutations in the MEFV gene located on chromosome 16p. The disease is characterized by recurrent episodes of serositis and fever which may be accompanied by an erysipelas-like rash.3,4 FMF is most prevalent in individuals of Ashkenazi Jewish, middle eastern, Turkish and north African descent, with an estimated disease rate in these populations of around one in five hundred and a carrier rate varying between one in eight to one in four.2 FMF has also been reported in populations, such as Greek and Italian, albeit at a much lower disease prevalence and carrier state.3 Our patient presented with a six-month history of intermittent right lower quadrant abdominal pain, joint pains and high grade fevers with suspicion for appendicitis requiring an appendectomy. Finally, pleuritic-like chest pain with associated marked troponemia and a technetium labeled WBC scanning revealing neutrophilic inflammation in the left ventricular myocardium and overlying pericardium.
Although myopericarditis is mainly caused by viral infections such as with coxsackie B, adenovirus, herpes viruses, hepatitis C virus, parvoviruses and echovirus, among others.4,5 in this case, it was most likely secondary to autoinflammation in the setting of FMF. The clinical presentation of myopericarditis is variable but commonly involves fever, fatigue, weakness, chest pain (usually pleuritic) and possibly a preceding flu-like viral illness. Due to the low yield and lack of targeted therapy, routine serologic testing for viral infections are not routinely performed.6,7 ECG findings of myopericarditis include transient ST segment and T-wave abnormalities in addition to atrial and ventricular tachyarrythmias.8-12 Early repolarization, atrioventricular and intraventricular conduction defects may be observed in myopericarditis.8-12 However, the absence of these ECG findings, as in our patient, does not rule out myopericarditis.12 Laboratory findings that may support the diagnosis of myopericarditis include an elevated white blood cell count, erythrocyte sedimentation rate, C-reactive protein and serum cardiac biomarkers such as troponin I and creatine kinase.8-10 The diagnosis is usually suggested with a combination of clinical symptoms, elevated inflammatory markers and cardiac biomarkers, typical ECG changes and possible echocardiographic evidence of left ventricular systolic dysfunction. However, other imaging studies (e.g. radionuclide scanning, cardiac MRI) can be used in the diagnosis.4-10,13
With regard to radionuclide scanning, gallium-67, indium-111, and technetium-99m have been used. Approximately 90 % of gallium-67 is retained in plasma after intravenous injection and almost all of it is bound to transferrin.12 Gallium-67 also binds to lactoferrin which has high levels in infectious foci.14,15 In myopericarditis, gallium-67 single photon emission commuted tomography (SPECT) scan usually reveals significant uptake in the myocardium and pericardium; however, use of gallium-67 over time has diminished because of its lack of specificity.14 WBC scanning to diagnose myopericarditis involves using the patient’s white blood cells, usually neutrophils, radiolabeled with technetium-99m or indium-111 antimyosin antibody and injected intravenously back into the patient, followed by single photon emission commuted tomography.15 As with the gallium scan, the WBC scan will usually reveal significant activity in the myocardium and pericardium on SPECT in patients myopericarditis.16 Both techniques of WBC scanning have been extensively used, specifically the indium-111 antimyosin antibody WBC scan. The sensitivity and negative predictive value of the indium-111 antimyosin antibody in diagnosing myocarditis were 91-100 % and 93-100 % in one study.17 The use of indium-111 antimyosin antibody scan has decreased over time because of radiation exposure and the requisite 48-hour delay in obtaining the imaging results.16 Our patient underwent a technetium-99m labeled WBC scan which revealed intense abnormal uptake in the left ventricular myocardium, with a noted small pericardial effusion.
More recently, cardiac MRI (CMR) has become a key component in the diagnosis of myocarditis.18 Possible findings in myopericarditis on CMR may include global left ventricular (LV) dysfunction or left ventricular wall motion abnormalities, pericardial effusion, transient increase in myocardial thickness, tissue edema, early gadolinium enhancement indicating hyperemia and capillary leakage, in addition to late gadolinium enhancement indicating necrosis and fibrosis.16 A definitive diagnosis of myopericarditis, which is unnecessary, would require endomyocardial biopsy (EMB), which is specific but not sensitive given the focal nature of the disease.4-10,13 We elected to forgo obtaining an endomyocardial biopsy in our patient since the workup that was performed was very suggestive of myopericarditis related to FMF and biopsy would not have altered management.
The mainstay of treatment of myopericarditis in the setting of FMF is to control the patient’s underlying autoinflammatory disease with NSAIDs and colchicine.4-10,13 Colchicine inhibits neutrophil chemotaxis, adhesion and mobilization by causing microtubule depolymerization.19 Colchicine also inhibits superoxide production in addition to NACHT-LRRPYD-containing protein 3 (NALP3) inflammasome and interleukin-1β (IL-1β).19 The MEFV gene, which is mutated in FMF, encodes pyrin, a protein involved in the regulation of inflammation.20 Normally, pyrin indirectly inhibits the formation of NALP3 inflammasome, but an abnormal pyrin protein is unable to carry out this function.21 Increased levels of IL-1β are also noted in monocytes carrying an MEFV gene mutation.21 This explains why colchicine, in addition to TNF-α and IL-1β antagonists can suppress inflammation in FMF.21
Our patient was a heterozygote for the E148Q MEFV mutation. V726A, M694V, M694I, M680I, and E148Q mutations account for the majority of all MEFV gene mutations, with the M694V mutation being the most prevalent mutation in Armenians, Arabs, Jews and Turks.22-24 Patients who carry one abnormal allele of the MEFV gene are predicted to be asymptomatic, but the FMF phenotype has been reported in heterozygotes.25 This finding postulates a more complex mode of inheritance.24-26
A definitive diagnosis of FMF based on the Tel-Hashomer clinical criteria requires two or more major criteria or one major plus two minor criteria (Table 2). A probable diagnosis requires one major criterion and one minor criterion.27 Our patient met two major criteria which included recurrent febrile episodes with serositis in addition to a favorable response to colchicine during attacks (Table 2). She was initially started on ibuprofen 400 mg BID for around 3 weeks after her episode of myopericarditis and was then transitioned to colchicine 0.6 mg daily which was titrated to 0.6 mg BID. In a small subset of patients who do not respond to colchicine, colchicine can be combined with either IL-1 inhibitory agents or TNF-α inhibitory agents in order to try to prevent the most feared complication of FMF, which is secondary amyloidosis.4-10,13 Disease control involves following the patient for recurrent “FMF attacks”, in addition to following inflammatory markers.
|
Major Criteria |
|
Recurrent febrile episodes accompanied by serositis |
|
AA amyloidosis without predisposing disease |
|
Favorable response to colchicine during attacks |
|
Minor Criteria |
|
Recurrent febrile episodes |
|
Erysipelas-like rash |
|
FMF in a first degree relative |
Table 2 Tel-Hashomer diagnostic criteria for familial Mediterranean fever (FMF)
The present study showed the pharmacological potential of the ethanolic extract of Neem bark. Our findings demonstrated that the F-EtOAc, obtained after saponification of EtCNeem, showed to be rich in phenolic and flavonoid compounds with antioxidant potential, as well as a nontoxic.
None.
None.

©2018 Assaf, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.