Journal of
eISSN: 2373-4396


Research Article Volume 9 Issue 5
1Cardiology Department, Khafr ELshiek Faculty of Medicine, Egypt
2Cardiology Department, Benha Faculty of Medicine, Egypt
3National Heart Institute, Egypt
Correspondence: Reda Biomy, Cardiology Department, Faculty of Medicine, Banha University, Borg Elesraa, Elgomhorya Street, Behind Elgomhorya Cinema, Kafr Elshiek, Egypt, Tel 1114383333
Received: July 21, 2017 | Published: September 20, 2017
Citation: Biomy R, Mansour H, Khaliek ELSA, Farghaly H, Mohamad S (2017) Short-Term Outcome of Balloon Angioplasty of Discrete Coarctation of Aorta. J Cardiol Curr Res 9(5): 00339. DOI: 10.15406/jccr.2017.09.00339
Introduction: Balloon angioplasty has been proposed as an alternative way to surgery in primary treatment of aortic coarctation (Co A) for both children and infants.
Objective: The purpose of this study is to assess the immediate and intermediate term effectiveness and safety of balloon angioplasty in infants and children with native discrete membranous Co A.
Patients and methods: Forty consecutive patients with native discrete coarctation of the aorta were treated with balloon angioplasty. They were 8 infants and 32 children; with ages ranged from 2 months to 10 years and all weighted below 35 Kg. All patients were clinically evaluated immediately after and at 6 to 12 months and 18 to 24 months after the procedure. Follow up data were collected and Doppler echocardiography was done before intervention to confirm diagnosis and to plan management and post interventional to follow-up patients and estimate the outcome and complications.
Results: Immediate successful relief of native Co A was obtained in 92.5% of the population study, while 7.5% recorded immediate suboptimal results with pressure gradient above 20mmHG. The peak systolic gradient across the coarcted segment was reduced significantly with 82.5% immediately after balloon angioplasty, 75% and 73.2% at the end of first and second follow up period respectively. Pressure gradient decreased significantly from 57±15.7 mmHg before balloon angioplasty to 14.23±11.30mmHg 9 to 12 months after, but it was increased slightly to reach 15.15±11.80 mmHg at the end of the second year. The systolic ascending aortic pressure decreased significantly (19%) and the systolic descending aortic pressure increased significantly (11.4%) at the end of the study. Recoarctation occurred in17.5% at the end of the 1st year and balloon angioplasty was repeated for all patients successfully. At the end of the 2nd year restenosis was evidenced in 10% of the whole study population. Aneurysm formation at the site of coarcted segment occurred in (5%).
Conclusion: Balloon angioplasty is an effective and safe alternative to surgery for treatment of Co A in infants older than 2 months and children10 years of age or younger.
Keywords: coarctation of the aorta, balloon angioplasty
Aortic Coarctation (CoA) is a congenital cardiac lesion consisting of a narrow aortic segment which constitutes 5 to 8% of all Congenital Heart Disease (CHD). The classic form of CoA is located in the descending thoracic aorta distal to the left subclavian artery origin, at nearly the level of the ductal structure. Significant systemic arterial hypertension and/or congestive heart failure are prompt indications of intervention. If hypertension (rather that heart failure) is the presenting problem, it is preferable to relieve the obstruction promptly rather that medical treatment of hypertension with antihypertensive medications. Surgical relief of the aortic obstruction and catheter interventional techniques (balloon angioplasty and stents) are available alternatives.1
Aortic coarctation includes a variety of lesions with variable degrees of hypoplasia of the aortic arch. There is either so severe aortic arch narrowing that is almost indistinguishable from interrupted aortic arch and arch hypoplasia, or on the other end of the spectrum the coarctation is discrete with a localized shelf-like lesion within the aortic arch lumen often with tapering of the arch as it courses towards the obstruction.2 There is more controversy about the optimal management of this vascular anomaly whether to balloon dilatation or stenting the native coarctation or operate upon, which surgical technique to select and the ideal age at intervention and the role of balloons and the different types of stents and the bioabsorbable stents for native and recurrent Coarctation.3 Despite the initial enthusiasm created by the effectiveness of balloon angioplasty in the treatment of aortic coarctation.4,5, the use of this technique became declined because the reported incidence of early recoarctation.6,7 and in particular, the aneurysm formation in the site of angioplasty.8 Despite these limitations, the use balloon angioplasty as the treatment of choice for localized coarctation have continued. The long-term follow-up studies of balloon angioplasty demonstrated favorable results which is comparable to results of surgery, as well as the absence of progression of the aneurysm in some cases.9,10 In addition, the increasing incidence of aortic aneurysms in patients with coarctation treated surgically, has kept the debate open regarding the proper treatment of aortic coarctation after the neonatal period.11
Although balloon angioplasty typically results in favorable acute results, it is associated with a higher rate of both recurrent obstruction and aortic wall injury than stent therapy. As a result, stent placement is usually preferred when patient size and coarctation anatomy are suitable.12 However, the use of conventional stents is restricted in newborns and young children because of continuous vessel growth, the possibility of redilatation, and later surgical removal. Several groups of researchers have used those techniques in the treatment of aortic coarctation in both children and adults. However, there is still a considerable controversy among cardiologist and surgeons with regard to whether the native aortic coarctation should be balloon dilated, stented or surgically treated.12 The objectives of this work are to share our experience, regarding both the results and the short and intermediate-term follow-up when using this technique; and to retrospectively compare our results with the previous results and complications of the balloon angioplasties done for coarctation of the aorta
Study population
Our study included 40 consecutive patients (8 infants and 32 children) with native discrete coarctation of the aorta referred for balloon angioplasty during a 2-years period from March 2013 to July 2015 at the National Heart Institute, Egypt. There were 27 males and 13 females; ages ranged from 2 months to 10 years (mean age is 3.6± 2.8); and all weighted below 35 Kg (mean weight is 16.2± 7.7). All parents or guardians of those children gave an informed consent and the study protocol was approved by the local ethic committee and all patients were followed for 18 to 24 months after balloon angioplasty.
Exclusion criteria includes
Previous aortic coarctation angioplasty or surgery, long aortic coarctation segment, severe aortic arch hypoplasia, associated abdominal aortic coarctation segment, associated complex cardiac anatomy and associated pathological extra cardiac syndromes.
Methods
Through history taking was done with special emphasis on history of hypertension (all patients were hypertensive for age), intermittent claudication, headache, chest pain, shortness of breath, and difficulty of feeding especially for infants and young children; special interest was directed for drug intake. Physical examination was done included Simultaneous palpation of the radial and femoral pulses and measurement of blood pressure in upper and lower limbs under basal conditions while the patient is recumbent and calm. Cardiac examination, conventional 12 leads ECG, chest x-ray were done. Doppler echocardiography were done for all patients. It was done as a pre-interventional investigation to confirm diagnosis and to plan management; then as a post interventional investigation to follow-up patients and estimate the outcome and detect any complications. Different views were made as long axis parasternal, short axis parasternal of the aortic, mitral valve and subvalvular mitral levels, apical four chambers views, subcostal and finally suprasternal views. M mode measurements in the parasternal long axis view were done for left ventricular end diastolic dimension (LEDD) and left ventricular end systolic dimensions (LVESD), septal wall thickness (SWT) and posterior wall thickness (PWT), Fractional shortening (FS) and ejection fraction (EF) were then calculated. Finally, in the suprasternal view; the aortic arch was delineated; the coarcted segment was determined by 2D and color Doppler, continuous wave Doppler study was done by alignment the cursor across coarcted segment to detect the peak systolic velocity and diastolic component. The ascending aorta, aortic arch and aortic isthmus were visualized using the suprasternal view. The transverse aortic arch diameter was measured at the base of the left common carotid and aortic Isthmus diameter was measured at the base of the left subclavian artery also the coarcted segment diameter measured.13
Cardiac catheterization
The procedure was performed under general anesthesia and arterial access was established through the right or left femoral artery using Seldinger technique. A 4 to 6 Fr introducer was selected, depending on the patient’s weight. Heparin was administered (100-150 IU/kg intravenously, with a maximum dose of 5000 IU), with activated clotted time monitored regularly and maintained at >200 s. Either pig-tail or multipurpose catheters were used, Generally, the pig-tail catheters were used for injections of dye and the multipurpose catheters were used for pressure measurement and for crossing the coarcted segment. Pull back peak to peak systolic pressures were measured in the aorta proximal to the coarcted segment and the descending aorta distal to the coarcted segment. Aortic arch injections were made using a non-ionic dye at a general dose not exceeding 4ml/kg for total injections. The anatomy of the transverse arch and the coarctation was defined by aortogram performed in the transverse aortic arch to show the narrowest part of the lesion in two different views. Angiographic projections included lateral and a left anterior oblique improved delineation of the lesion if the isthmus overlaps the descending aorta. A pigtail catheter with multiple markers or an angiographic catheter with calibration marks was used for accurate measurement of the diameter and length of the coarctation, the diameters of the transverse arch between the brachio-cephalic and the left common carotid artery, isthmus just after the origin of the subclavian artery, aorta proximal and distal to the coarctation and the aorta at diaphragm level.
The origins of the brachiocephalic, carotid, and subclavian arteries are noted and irregularities or aneurysms of the aortic wall are carefully excluded. Those measurements were related to the size of the catheter used to avoid magnification errors. A long stiff 0.035-inch exchange guide wire was placed through the retrograde injection catheter, with the tip in a secure position, either the left subclavian artery if it arises some distance away from the coarctation or has a large diameter, then it is the best option for anchoring the guide wire. If not, other alternatives are the ascending aorta or the right subclavian artery, the latter allowed for a straight and secure position of the guide wire. The tip of the guide wire was always kept away from the coronary, carotid, or vertebral arteries. The arterial introducer sheath was replaced by a larger size, if necessary, to accept the balloon selected. It was preferable to use a slightly larger vascular sheath than a balloon catheter inserted directly over a wire, which avoid the risk of arterial damage. The diameter of the initial balloon chosen for the procedure was equal to or 1 mm smaller than the diameter of the aorta immediately distal to the origin of the subclavian artery, and not exceeding the diameter of the aorta at the diaphragm. The shortest possible balloons for the specific anatomy were selected, covering the entire length of the coarctation with small shoulders. The balloon is advanced to just cross the coarctation, it is then centered at the coarctation level and inflated using a pressure-monitored inflation device till 5 atmospheric pressures, or until the waist disappears.
The inflation/deflation times were not more than 10 sec Balloon inflation is then repeated two to four times. The balloon was kept away from the carotid and vertebral arteries to minimize the risk of trauma. Once the coarctation has been dilated, the balloon is removed and a multipurpose catheter attached to a valve or hemostatic adaptor, or a Multi-Track TM catheter, is used. Gradients are recorded while crossing the coarctation area, maintaining the guide wire in position. Final angiograms in two different image views are obtained to detect any immediate complications (e.g. newly developed aneurysm or dissection), then pressure measurements were then repeated. The guide wire had not been withdrawn across the dilation site until the procedure is complete. A pigtail catheter was only removed over a guide wire, avoiding potential contact of the free tip with an intimal tear, which could cause additional trauma to the arterial wall. If there is still angiographic narrowing at the coarctation site or a significant residual peak systolic gradient is seen, and the aortic wall shows no damage, a balloon 1 to 2 mm larger may be used, not exceeding 10–15% of the normal size of the adjacent aorta, and not exceeding the diameter of the aorta at the diaphragm. Balloon angioplasty was considered successful when the post dilatation pressure gradient was <20 mmHg and the coarctation diameter was increased without formation of a significant aneurysm on repeat angiography (An aneurysm was defined as a saccular outpouching protrusion of the aortic wall or fusiform dilatation with a ratio of its diameter to the descending aorta diameter more than 1.5 at follow-up).14
Follow-up: All patients underwent periodic clinical evaluation immediately after and at 6 to 12 months and 18 to 24 months. Follow-up data were collected consisting mainly of history taking, cardiovascular examination with special emphasis to measurement of upper and lower limb blood pressure; blood pressure was measured in the right arm and in the leg contralateral to the side used for angioplasty. Doppler echocardiography was done to demonstrate the specific changes in the site of obstruction, to determine the cardiac functions and to detect any complications (specially recoarctation and aneurysm). While pulsed and continuous-wave Doppler study used to determine the pressure gradient. Balloon angioplasty was considered successful when the coarctation diameter was significantly increased and the post dilatation pressure gradient was ≤20 mmHg measured by cuff sphygmomanometer, echocardiographic Doppler-derived peak pressure gradient or at repeat cardiac catheterization if needed, without formation of a significant aneurysm.
An aneurysm was defined as a saccular outpouching protrusion of the aortic wall or fusiform dilatation with a ratio of its diameter to the descending aorta diameter more than 1.5 folds.15
Statistical analysis
The data was collected, coded and entered to a personal computer. It was analyzed with the program Excel 2016. Values are expressed as (mean ± standard deviation). The student paired t-test was used to compare pre- and post angioplasty pressure gradients. A p value less than 0.05 was considered significant.
In this study the commonest associated lesion found was bicuspid aortic valve with no significant pressure gradient in 8 patients (20%), followed by aortic valve stenosis in 7patients (17.5%) with no significant pressure gradient. Regarding history and clinical examination all patients were hypertensive for age, but only 14 patients (35%) were on anti-hypertensive and /or anti-failure treatment. Headache (7%), shortness of breath (14%), and difficulty of feeding especially for infants and young children was observed in 12 patients (30%). In all patients, the pulse in the upper limbs were felt while it was weak and delayed in the lower limbs. The systolic and diastolic blood pressure in the upper limbs ranged from 190 to 120 (152.8±14.2) and from 120 to 60 (90.1±13) respectively. The systolic and diastolic blood pressure in the lower limbs ranged from 120 to70 (96.6±12.4) and from 100 to 50 (72.4±14.9) respectively. All patients had an arm to leg-cuff measured systolic pressure gradient ≥20 mmHg. The electrocardiogram showed left ventricular hypertrophy in 15 patients almost with strain pattern (37.5%).
Doppler echocardiography data before angioplasty: LVEDD ranged from 2.2 to 5.1 cm (3.42±0.51), LVESD ranged from 1.4 to 4 cm (2.33±0.52). SWT ranged from 0.5 to 1.2 (0.74±0.16) and the PWT ranged from 0.5 to 1 (0.7±0.12). The fractional shortening (FS) ranged from 11% to 48% (33.3±8.41); and the ejection fraction (EF) ranged from 28% to 79% (64.0±11.68); The Doppler pressure gradient ranged from 30 to 100 mmHg (57±15.7). Left ventricular concentric hypertrophy was present in 23 patients (57.5%).
Cardiac catheterization hemodynamic and angiographic data before angioplasty: The measured PG across the coarcted segment ranged from 25 to 85 mmHg (51.97±15.2 ). The systolic and diastolic ascending aortic blood pressures were 125 to 195 mmHg (157.1±17.9 ) and 55 to 125 mmHg (92.75±16.60), respectively. The systolic and diastolic descending aortic pressures were 70 to 140 mmHg (103.75±16.16) and 40 to 85 mmHg (74.625±15.91) respectively (Table 1).
|
Before Angioplasty |
Immediately after |
T test P |
|
|
PG (mmHg) |
51.97±15.24 |
9.075±8.71 |
<0.001 |
|
AASP (mmHg) |
157.1±17.97 |
129.03±11.99 |
<0.001 |
|
AADP (mmHg) |
92.75±16.60 |
85.2±10.24 |
<0.001 |
|
DASP (mmHg) |
103.75±16.16 |
119.65±10.23 |
<0.001 |
|
DADP (mmHg) |
74.625±15.91 |
81.2±9.36 |
<0.001 |
Table 1 Comparison between cardiac catheterization hemodynamic data before and immediately after balloon angioplasty
PG, pressure gradient; AASP, ascending aorta systolic pressure; AADP: ascending aorta diastolic pressure; DASP: descending aorta systolic pressure; DADP: descending aorta diastolic pressure
The transverse aortic arch ranged from 0.82 to 1.3 cm (1.06±0.15), the isthmus ranged from 0.63 to 1.3 cm (0.91±0.18),the coarcted segment diameter ranged from 0.2to 0.75cm (4.39±1.6), The descending aortic diameter ranged from 0.7 to 1.5 cm (1.14±0.19) (Figure 1).
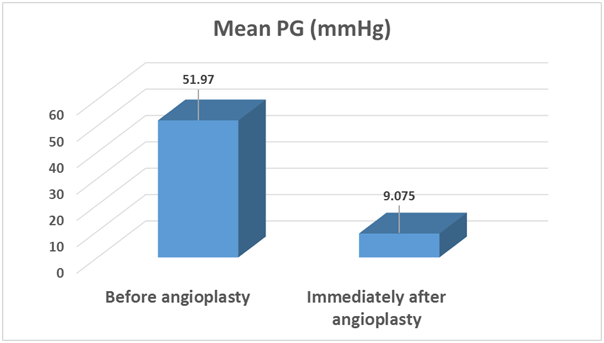
Figure 1 Comparison between pressure gradient across coarcted segment before and immediately after balloon angioplasty.
Immediate hemodynamic results: The peak to peak systolic gradient significantly decreased from 51.97±15.24 to 9.075±8.71mmHg (P<0.001) with 82.7% reduction. The systolic ascending aortic pressure significantly decreased from 157.1±17.97 to 129.03±11.99 mmHg (P<0.001) and the systolic descending aortic pressure significantly increased from 103.75±16.16 to119.65±10.23 mmHg (P<0.001). Thirty-seven angioplasty procedures were considered to be immediately successful in 92.5% of patients. Three patients (7.5%) had suboptimal immediate results with their recorded pressure gradient 25, 30,40 mmHg. All but 3 patients were discharged 24 hours after balloon angioplasty.
Immediate angiographic results: The mean diameter of the coarcted segment doubled significantly from 0.439±0.16 to 0.87±0.16 cm (P<0.001); and the isthmic diameter also significantly increased from 0.91±0.18 to0.96±0.18 cm (P<0.001) (Table 2). Immediately after balloon angioplasty a small aneurysm appear in 2 patients the diameter of both didn’t exceed double diameter of the descending aorta.
|
Before Angioplasty |
Immediately after Angioplasty |
T test P |
|
|
TA Diameter (cm) |
1.06±0.15 |
1.08±0.16 |
0.168 |
|
Isth Diameter (cm) |
0.91±0.18 |
0.96±0.18 |
0.0008 |
|
CoA seg Diameter (cm) |
0.44±1.6 |
0.87±1.63 |
0.0007 |
|
DA Diameter (cm) |
1.14±0.19 |
1.12±0.21 |
0.241 |
Table 2 Comparison between cardiac catheterization angiographic data before and immediately after balloon angioplasty
TA, transverse arch; Isth, isthmus; CoA seg, coarctation segment; DA, descending aorta
Follow up
9 to 12 months after balloon angioplasty; clinically almost all symptoms were resolved; only 2 patients (5%) still suffer of shortness of breath; while no patient suffer of that symptom at the 18 to 24 months (Table 3). Mean systolic blood pressure in the upper limb decreased from (152.8±14.2) to (129.13±14.84) at the end of the 1st follow-up period and to (124.25±15.38) at the end of the 2nd follow-up period. Mean systolic blood pressure in the lower limb increased from (96.6±12.4) to (117.38±11.6) at the end of the 1st follow-up period and to (112.25±12.81) at the end of the 2nd follow-up period (Table 4 & 5).
|
Symptoms |
Pre-angioplasty |
9 to 12 Months follow-up |
18 to 24 Months follow-up |
|
Difficulty in Feeding |
12 patients |
0 patients (0%) |
0 patients (0%) |
|
Intermittent Claudication |
0 patients |
0 patients (0%) |
0 patients (0%) |
|
Shortness of Breath |
16 patients |
2 patients |
0 patients (0%) |
|
Hypertension |
40 patients |
12 patients |
3 patients (7.50%) |
|
Headache |
7 patients |
0 patients (0%) |
0 patients (0%) |
|
Chest Pain |
0 patients (0%) |
0 patients (0%) |
0 patients (0%) |
Table 3 Symptoms at follow up in comparison to pre-angioplasty status
|
Before Balloon |
9 to 12 Months after Balloon (Mean±SD) |
T test P |
|
|
ULSBP |
152.8±14.2 |
129.13±14.84 |
<0.001 |
|
ULDBP |
90.1±13 |
83.5±10.51 |
0.003 |
|
LLSBP |
96.6±12.4 |
117.38±11.60 |
<0.001 |
|
LLDBP |
72.4±14.9 |
80.25±10.91 |
0.006 |
Table 4 Comparison between blood pressure measurement before angioplasty and 9 to 12 months after the procedure
|
Before Balloon |
18 to 24 Months after Balloon (Mean±SD) |
T test P |
|
|
ULSBP |
152.8±14.2 |
124.25±15.38 |
<0.001 |
|
ULDBP |
90.1±13 |
81.75±10.53 |
<0.003 |
|
LLSBP |
96.6±12.4 |
112.25±12.81 |
0.26 |
|
LLDBP |
72.4±14.9 |
77.13±6.59 |
0.006 |
Table 5 Comparison between blood pressure measurement before angioplasty and 18 to 24 months after the procedure
ULSBP, upper limb systolic pressure; ULDBP, upper limb diastolic pressure; LLSBP, lower limb systolic pressure; LLDBP, lower limb diastolic pressure
Follow-up by echocardiography showed decrease in PG from (57±15.7) at the start of the study to (14.23±11.3) at the end of the 1st follow-up period and to (15.15±11.8) at the end of the 2nd follow-up period.
Echocardiography also showed increase in coarctation diameter from (04.2±1.7mm) at the start of the study to (7.98±2.35 mm) at the end of the 1st follow-up period and to (8.7±2.27mm) at the end of the 2nd follow-up period (Table 6 & 7). At the end of the 1st follow-up period the Recoarctation incidence reached 17.5% (7 patients) and at the end of the 2nd follow-up period 4 patients (10%) showed evidence of Recoarctation.
|
Before Balloon Angioplasty (Mean±SD) |
After 9 to 12 Months (Mean±SD) |
T test P |
|
|
LVEDD (cm) |
3.42±0.51 |
3.29±0.29 |
0.043 |
|
LVESD (cm) |
2.33 ±0.52 |
2.15±0.3 |
0.122 |
|
FS |
33.3±8.41 |
34.86±4.95 |
0.285 |
|
EF |
64.0±11.68 |
70.56±4.5 |
0.091 |
|
IVS (cm) |
0.74±0.16 |
0.73±0.121 |
0.42 |
|
PW (cm) |
0.72±0.12 |
0.73±0.11 |
0.031 |
|
AA diameter (cm) |
1.13±0.23 |
11.38±2.26 |
0.031 |
|
Isth diameter (cm) |
1.01±0.26 |
10.5±2.52 |
0.0038 |
|
CoA seg. diameter (cm) |
0.42±0.17 |
7.98±2.35 |
0.0041 |
|
Doppler PG (mmHg) |
57±15.7 |
14.23±11.30 |
0.24 |
Table 6 Comparison between Doppler echocardiographic parameters before balloon angioplasty and 9 to 12 months’ follow-up
|
After 9 to 12 Months |
After 18 to 24 Months |
T test P |
|
|
LVEDD (cm) |
3.29±0.29 |
3.83±0.44 |
0.056 |
|
LVESD (cm) |
2.15±0.3 |
2.22±0.29 |
0.024 |
|
FS |
34.86±4.95 |
35.62±5.17 |
0.14 |
|
EF |
70.56±4.5 |
70.3±3.85 |
0.34 |
|
IVS (cm) |
0.73±0.121 |
0.75±0.12 |
0.29 |
|
PW (cm) |
0.73±0.11 |
0.73±0.11 |
0.42 |
|
AA diameter (mm) |
11.38±2.26 |
11.68 ±1.85 |
0.03 |
|
Isth diameter (mm) |
10.5±2.52 |
10.9 ±2.12 |
0.003 |
|
CoA seg. diameter (mm) |
7.98±2.35 |
8.7±2.27 |
0.004 |
|
Doppler PG (mmHg) |
14.23±11.30 |
15.15±11.80 |
0.24 |
Table 7 Comparison between Doppler Echocardiographic parameters 9 to 12 months and 18 to 24 months’ follow-up after balloon angioplasty
LVEDD, left ventricular end systolic diameter; LVESD, left ventricular end diastolic diameter; FS, fraction shortening; EF, ejection fraction; IVS, interventricular septum; PW, posterior wall; AA, aortic arch; Isth, isthmus; CoA, coarctation segment
Medical treatment
In this study, 86% (12/14) patients who were taking anti-failure and/or antihypertensive medication (as angiotensin converting enzyme inhibitors-ACE, beta blockers and diuretics) could stop them with amelioration of symptoms at the end the 2nd year of follow up.
Doppler echocardiography
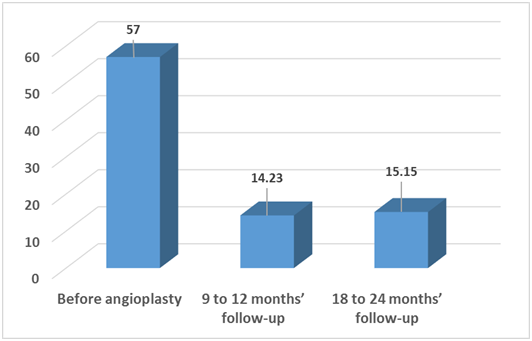
Figure 2 Comparison between pressure gradient across the coarcted segment diameters before angioplasty, 9 to 12 months and 18 to 24 months follow up periods.
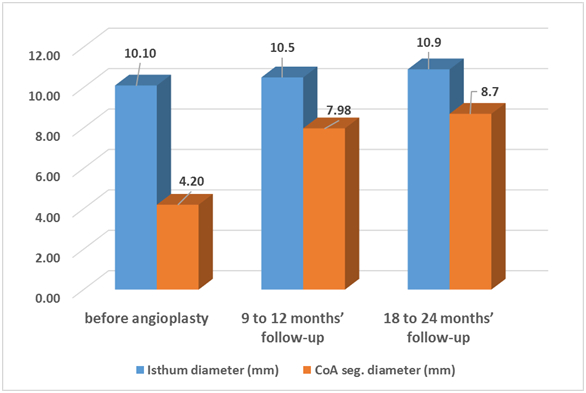
Figure 3 Comparison between isthmus and coarcted segment diameters before angioplasty, 9 to 12 months and 18 to 24 months follow up periods.
Complication: There was no patient mortality related to the procedure within about 24 months following balloon angioplasty. Three patients (7.5%) which had reduced pulses in the catheterized leg improved later. Blood loss requiring blood transfusion occurred in another three.
Early Failure: Three patients showed suboptimal initial procedure with a maximum systolic pressure gradient of more than 20 mmHg. Re-catheterization and dilation was done; which was successful in two patients.
Aneurysm formation: Two patients (5%) had small aneurysms at the site of coarctation diagnosed by angiography immediately after balloon angioplasty.
Recoarctation: The recoarctation incidence reached 17.5% in the whole studied population (7/40 patients) about the end of the 1st year follow-up. All the patients had successfully repeated angioplasty with post dilatation gradient ranging from 0 to 10 mmHg and 3 of them maintained no clinical evidence of restenosis during their second follow-up period while 4 patients (10%) developed evidence of restenosis during their second follow-up period.
Immediate results: Immediate successful relief of native coarctation was obtained in 92.5% of our population study. While 7.5% of the patients recorded immediate suboptimal results with PG above 20 mmHg. By the end of the study, 87.5% of our patient population have PG less than 20 mmHg across the coarcted segment (35 patients); 10% show evidence of recoarctation (4 patients) while the procedure failed in 1 patient. The peak systolic gradient across the coarcted segment was reduced significantly with 82.5% reduction in pressure gradient immediately after balloon angioplasty measured in cath lab, 75% and 73.2% at the end of 1st and 2nd follow up period respectively recorded by echocardiography and, as a result of the procedure, the systolic ascending aortic pressure decreased significantly (19%) and the systolic descending aortic pressure increased significantly (11.4%) at the end of the study.
Intermediate results about two-years follow-up: The late results of balloon angioplasty are considered good when residual pressure gradient was reduced to ≤20 mmHg measured by cuff sphygmomanometer, Doppler-derived peak instantaneous PG, cardiac catheterization as well as the absence of restenosis and no significant aortic aneurysm on follow-up.16
The Doppler measured pressure gradient decreased significantly from (mean ± SD) 57±15.7 mmHg before balloon angioplasty to 14.23±11.30mmHg (p=0.003) after 9 to 12 months but it increased slightly to reach 15.15±11.80 mmHg (p=0.002) at the end of the second year of follow up. This is similar to reports published by other researchers. Lan He et al.17, reported that the systolic peak pressure gradient (PG) across the coarctation was 41.0±16.0 mmHg (range 13-76 mmHg) which was significantly decreased to 13.0±11.0 mmHg (range 0-40 mmHg) and Massoud et al.15, reported that peak-to-peak systolic gradient decreased from 55.65 ± 15 to 9.6 ± 8 mmHg (p<0.0001).15
Recoarctation
In this study recoarctation incidence reached 17.5% in the whole studied population (7/40 patients) about the end of the 1st year follow-up, but it was up to 25% among infants aged one year or less (2/8 patients). All the 7 children (6 of them were male children) had successful repeat angioplasty with post dilatation gradient ranging from 0 to 10 mmHg and 3 of them maintained no clinical evidence of restenosis during their second follow-up period while 4 patients (10%) showed evidence of restenosis during their second follow-up period.
The relatively lower recoarctation rate met in our study could be explained by the low percentage of infant age group (one year or less) as they consist 20% only of the studied population (8/40 children). In other studies, when series include neonates and infants, the incidence of recoarctation is higher. The population in the Rao et al..18 study, ranged from 2 days to 15 years. At a mean follow up period of 14±11 months. They showed 25% recoarctation rate. (83% in neonates less than 30 days old, 39% in infants less than 1 year old, 8% in children). Several risk factors exist for recoarctation after balloon angioplasty, including age at initial repair < 2-3 months, weight at initial repair < 5 kg, size of aortic isthmus (isthmic hypoplasia), small coarcted segment diameter.19
Aneurysm formation
In the present study, aneurysm at the site of coarctation developed in 2 patients (5%) diagnosed by angiography immediately after balloon angioplasty. This was comparable to Massoud et al.15, who reported in their study that the incidence of immediate aneurysm formation was 6.5%. The pathological basis for aneurysm formation may be similar to the mechanism by which balloon angioplasty relieves coarctation, namely controlled tearing of aortic intima and media. Other additive factors may contribute to the formation of aneurysm at the site of balloon dilatation such as, the use of larger balloons or inherited wall abnormalities (cystic medial necrosis). This is found in the form of severe depletion and disarray of elastic tissue. It is not clear, whether those factors could act independently or in contribution to produce such aneurysm.20
In our study the total number of patients suffering from complications is 8 out of 40 patients (20%).No one suffered from life threatening incidents or major complications related to the procedure or requiring immediate surgical intervention. Three patients (7.5%) suffered from blood loss during angioplasty requiring blood transfusion. Three patients (7.5%) suffered from peripheral vascular complications and were treated by IV low molecular weight heparin and were discharged at the same week. Two patients (5%) suffered from in situ aneurysm formation and one patient (2.5%) suffered from suboptimal results in spite of 3 repeated angioplasty procedures throughout the study duration.
Medical treatment
The treatment of hypertension in coarctation of the aorta is ideally managed by early surgical or trans catheter repair to reduce the risk of irreversible aortopathy and refractory hypertension, in addition to other associated squeals including premature coronary artery disease; stroke; aortic aneurysm, dissection, and rupture; infective endocarditis; and heart failure. In a large follow up study including 646 patients with aortic coarctation, it was found that hypertension occurred in 7% of patients operated on as infants, as opposed to 33 % of patients who had repair performed after the age of 14 years; The study also found age at the time of initial repair to be the most important predictor of hypertension.21 In our study, 85.7% (12/14) patients who were taking both anti-failure and antihypertensive medication could stop them with amelioration of symptoms at the end the 2nd year of follow up. By the end of the study 95% (38/40) of patients were not taking any medical treatment and the patients who were still on antihypertensive and anti-failure medications were referred to surgery.
Massoud et al.15 reported that almost all the children studied stopped medication by the end of 24-60month follow up period except for three who were also referred to surgery due to aneurysms. Another 4 patients (all children) had a cuff pressure gradient of 20–22 mmHg with hypertension, without angiographic evidence of restenosis associated with persistent mild isthmus hypoplasia; they received atenolol and captopril.15
Percutaneous balloon angioplasty was still found to represent an effective and successful mean of relieving obstruction due to native discrete aortic coarctation in infants older than 2 months and children 10 years of age or less.
None.
Authors declare that there is no conflict of interest.

©2017 Biomy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.