Journal of
eISSN: 2373-4396


Mini Review Volume 9 Issue 5
King Saud Bin Abdulaziz University for Health Sciences, Saudi Arabia
Correspondence: Mohammed Balghith, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, P O Box 24490, Saudi Arabia, Tel 966504147204
Received: September 08, 2017 | Published: September 19, 2017
Citation: Balghith M (2017) Update in TAVR. J Cardiol Curr Res 9(5): 00338. DOI: 10.15406/jccr.2017.09.00338
Surgical aortic valve replacement (SAVR) has been the gold standard therapy for severe aortic stenosis. However, transcatheter aortic valve replacement (TAVR) is now generally accepted as the new standard of care for patients with symptomatic aortic stenosis who are not suitable for open surgery.1 Currently TAVR may also be a preferred alternative to SAVR in highly selected high-risk, but still operable, patients in whom morbidity and mortality may be reduced. Although TAVR outcomes continue to improve, concerns remain with respect to vascular injury, stroke, paravalvular regurgitation, and valve durability.2
However, it seems likely that with ongoing refinement of transcatheter valve systems, techniques, and patient selection TAVR is becoming an increasingly appealing option for a much broader range of patients. Randomized trials and ongoing surveillance will play an important role as we enter a new era of rigorous clinical evaluation for minimally invasive therapies for structural heart disease. It is considered the procedure of choice according to the current trials and guidelines, mainly in high risk patients with severe aortic stenosis.3
The development of heart team approach in most of the hospital around the world lead to recruiting of more patients who are in need for this technique. In the last few years there has been a lot of advancement in the technology of TAVR, in addition to a rapid evolution from first generation (The Edwards SAPIEN TAV balloon-expandable valve (Edwards Lifesciences, Irvine, California), (The CoreValve ReValving System (Medtronic Inc., Minneapolis, Minnesota) utilizes a self-expanding nitinol, to second and third generation of TAV. There has also been a change of practice recently from transapical to Percuataneous Transfemoral implantation, also sublavian and even transcarotid can be tried in some the difficult access patients .4
Transcaval TAVR is another new approach used for aortic valve replacement. Percutaneous transcaval TAVR was first performed in Europe using an expandable introducer sheath for the implantation of Edwards SAPIEN 3 aortic valve. Due to severe peripheral artery disease, chronic obstructive pulmonary disease, and renal insufficiency, the patient was not considered a candidate for transfemoral or transapical treatments. Once the eSheath (Edwards Lifesciences, Irvine, CA, USA) was introduced into the abdominal aorta via the femoral and inferior vena cava, TAVR was initiated in accordance with standard procedures. Based on these results, the study concluded that transcaval venous access to the aorta could be a new strategy for TAVR in otherwise ineligible patients as a safe approach using expandable sheath technology.5
The drawbacks of TAVR were mainly, paravalvular leak, heart block and the need for permanent pacemaker implantations, vascular complications and strokes.6,7 The PVL is due to multiple mechanisms during TAVR, therefore this problem has been further addressed with the development of completely recapturable valves that can be exchanged for larger valves if needed. Malposition is not a common problem but should be eliminated by the current generation of self-expanding and expandable valves that allow recapture, reposition, and redeployment before release. The final issue of patient anatomy is more complex. Repositionability will help by allowing the implanting physician to choose the optimal landing site for the valve, which is not always obvious on the first attempted deployment. Additionally, valves have been developed with sealing skirts to fill the uneven spots along the landing zone, which helps to eliminate PVL (Figure 1 & 2).
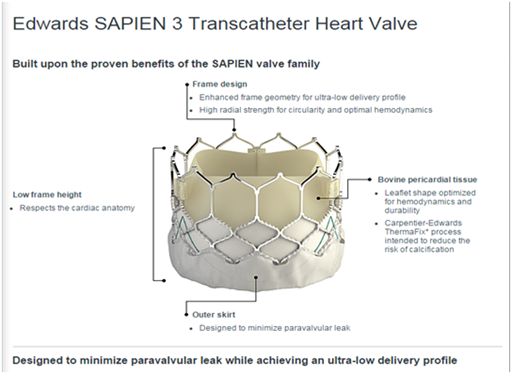
Figure 1 Edwards Sapien 3 valve showing the outer skirt, which designed to decrease the paravalvular leak.
The vascular complications was reduced since the companies introduced a small French sheaths through the femoral approaches, both Medtronic and Edwards introduced a 14 F femoral sheaths for even smaller access of 5mm (Figure 3 & 4). These data from U.S. pivotal trial for these devices, PARTNER 1 parts A and B, demonstrate excellent durability of transcatheter heart valves, suggesting that the low 5-year survival observed in this cohort is not related to adverse hemodynamics or transcatheter heart valve deterioration, and TAVR Durability: Some Reassurances From CoreValve Trials Out to 9 Years from EuroPCR 2017.8
The Valve in Valve (VIV) treatment for failed previous surgical valves (Figure 5), is moving forward and recently the TAVR was approved by FDA as a treatment option for failed aortic valve bioprosthesis.9 The rare complications like valve thrombosis and endocarditis are still a concern. With good early clinical suspecion in addition to meticulous echocardiogram and advanced CT scanning, this will lead to correct diagnosis and prompt treatment.10,11 Recently, the usage of TAVR in intermediate and low risk aortic stenosis patients is becoming more popular with almost equal or even superior results than SAVR.12
None.
There were no financial interest or conflict of interest.

©2017 Balghith. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.