Journal of
eISSN: 2373-4396


Case Report Volume 9 Issue 1
University of Miami, Miller School of Medicine, USA
Correspondence: Marcelo Fernandes, University of Miami, Miller School of Medicine, USA, Tel (864) 903-9621
Received: June 01, 2017 | Published: June 6, 2017
Citation: Fernandes M, Grevious S, Martinez C (2017) ST-Segment Elevation during Dobutamine Stress Echocardiography in a Patient with Normal Coronary Arteries. J Cardiol Curr Res 9(1): 00311. DOI: 10.15406/jccr.2017.09.00311
segment elevation, echocardiography, sustained ventricular tachycardia, intracranial hemorrhage, myocardial infarction, hypothyroidism
ST, segment elevation; DSE, dobutamine stress echocardiography; CAD, coronary artery disease; NO, nitric oxide
Dobutamine stress echocardiography (DSE) is an effective modality for determining the presence of myocardial ischemia from obstructive coronary artery disease (CAD).1 Several large studies have deemed DSE a largely safe imaging tool, with minimal side effects.2‒5 The most serious and rare side effects being sustained ventricular tachycardia, intracranial hemorrhage, and myocardial infarction.5‒8 Within the subset of myocardial infarction events, there have been extremely rare cases in the literature of transmural myocardial infarction with development of ST-segment elevation, occurring in about 0.1% of reported cases.3,9‒11 ST-segment elevation during DSE has been commonly associated with significant luminal stenosis and subsequent coronary artery disease.9,12‒15 ST-segment elevation without evidence of critical luminal lesions is a significantly uncommon event.13 We report the case of a patient who developed transient ST-segment elevations during DSE.
A 79year-old woman with hypothyroidism but no further cardiac risk factors was referred for a pre-operative evaluation given her poor functional capacity. The patient underwent a pharmacologic stress echo with dobutamine given her inability to exercise secondary to a prior knee operation and back pain. Dobutamine was infused at 3-minute intervals, starting with 10ug/kg and increasing to 20ug/kg, 30ug/kg, and 40ug/kg until a maximum heart rate of 129bpm was achieved, accounting for 91% of her max predicted heart rate. Patient was asymptomatic with no ST-segment changes at peak stress and without evidence of wall motion abnormalities. During the early recovery phase, 1.5-2.5mm ST-segment elevations in leads II, III, aVF, and V3-V6 (Figure 1) on continuous ECG monitoring were noted. While the patient had no anginal symptoms, she complained of dyspnea and developed systemic hypotension with a blood pressure of 75/40mmHg. She was loaded with aspirin 325mg and resuscitated with intravenous saline administration. Her blood pressure normalized, and, given persistent ST-segment elevation, she was emergently taken to the catheterization laboratory for further invasive evaluation. Coronary angiography showed non-obstructive coronary artery disease, with mild irregularities of the proximal to mid LAD (Figure 2), with no evidence of thrombus, myocardial bridging, or coronary vasospasm. There was no evidence of obstruction to the RCA (Figure 3). No vasospasm challenge was performed in the cath lab. Her ejection fraction on venticulogram was estimated at 60%-65%, with no wall motion abnormalities or significant mitral insufficiency noted. Her troponins remained normal, and the patient was discharged in stable condition on aspirin, beta-blocker, and calcium-channel blockade.
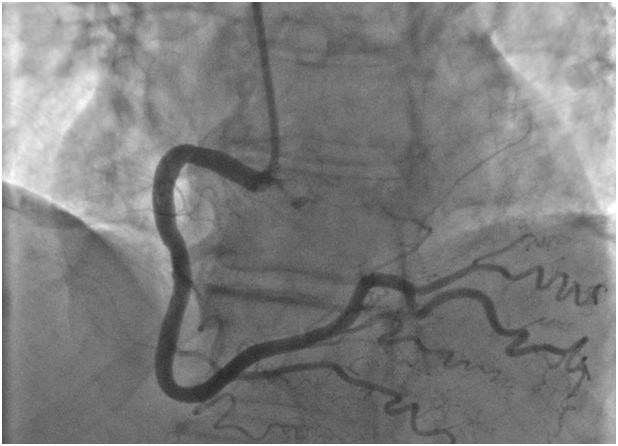
Figure 3 Coronary angiogram in LAO Cranial view of the right coronary artery showing normal coronary.
Dobutamine is a strong β-1 receptor agonist, with modest activity at α-1 and β-2 receptors. Its agonist activity at the β-1 receptor increases heart rate, and as such pharmacologic stress protocols in patients unable to exercise. It is best when accompanied by an imaging protocol such as echocardiography, which allows for the evaluation of segmental wall motion under pharmacologic stress protocols. DSE has been shown to be a safe procedure for routine use.1 However, rare cases of ST-elevation myocardial infarction have been described, with cases involving significant luminal stenosis on angiography and in those without evidence of obstructive CAD,11,13‒18 the latter being a more rare event. In addition to increasing myocardial oxygen demand by its activity at the β1 receptor and increase in heart rate, dobutamine may also potentially induce coronary artery vasospasm. The incidence of DSE-induced vasospasm has been reported to range between 0.14% - 0.4%.10,13 The mechanism behind this phenomenon is felt to be related to the α-1 agonist activity of the medication which can lead to a paradoxical increase in α-1 activity in mildly diseased vessels, causing vasoconstriction and vessel spasm.3 In patients with underlying coronary spastic angina, dobutamine loses its dilator effect on the endothelium, and, instead, vasoconstriction predominates, leading to spasm and subsequent ST-elevation without evidence of coronary stenosis.18 It is well understood that there is a basal release of endothelial nitric oxide (NO) to maintain proper basal tone of the coronary arteries.19,20 However, there is a deficiency in endothelium release of basal NO in the coronary arteries of patients with underlying coronary spastic angina.21 Investigators postulate that as consequence of dobutamine led increased flow in diseased vessels, there is a subsequent increase in shear stress in the vessels, leading to both nitric oxide and free radical production. The latter interferes with endothelium dependent vasodilation, and thus, during DSE, dobutamine’s dilator properties are lost in favor of predominantly vasoconstriction forces in patients with underlying coronary spastic angina.18 Dobutamine can uncover true vasospastic angina as well as induce coronary vasopasm in patients without underlying vasospastic angina.13 We did not perform provocative coronary spasm testing in our patient as current international guidelines indicate that provocative testing in patients without prior symptoms suggestive of vasospatic angina is contraindicated,22 and thus would not have been appropriate in our case. Therefore, we cannot conclusively state whether DSE simply unmasked a previously undiagnosed propensity for vasospastic angina or if the coronary spasm was a direct idiosyncratic side effect of DSE itself. Although given the fact the patient had no prior clinical history of variant angina, we hypothesize that it was likely more of the latter. In our case, this occurred in the recovery phase, and not immediately with drug infusion, suggesting there may be some delayed effect. While DSE has a favorable safety profile and low overall risk, rare complications do occur. In addition to the small risk of myocardial ischemia/infarction inherent in increasing myocardial oxygen demand in a population of patients with established underlying CAD, the idiosyncratic secondary side effects of dobutamine may also provoke coronary spasm, though the use of dobutamine as an agent for provocative testing has not been fully investigated. However, if sustained and untreated vasospasm occurs, it can potentially lead to myocardial injury even in a population of patients without obstructive CAD. Therefore, close monitoring is imperative both during and in the immediate post-procedure period.
None.
None.
Author declares there are no conflicts of interest.

©2017 Fernandes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.