Journal of
eISSN: 2373-4396


Case Report Volume 8 Issue 2
1Cardiology, Hospital Espa
2Cardiology, Mexican Institute of Transplantation, Mexico
Correspondence: Lainez Zelaya J, Cardiology, Mexican Institute of Transplantation, Morelos, Mexico, Tel 52 044 55 6421 0039
Received: January 26, 2017 | Published: February 1, 2017
Citation: Berrios-Barcenas E, Islava-Gálvez and Laínez-Zelaya J (2017) Risk Stratification in Frequent Ventricular Extrasystoles: The Importance of Cardiac Magnetic Resonance. J Cardiol Curr Res 8(2): 00273. DOI: 10.15406/jccr.2017.08.00273
Ventricular tachycardia (VT) has a direct relationship with sudden cardiac death (SCD) and risk stratification is essential for further treatment. In a context of a structural normal heart, idiopathic VT is the principal suspicion. Cardiac magnetic resonance (CMR) is a powerfull tool to evaluate the anatomic substrate of ventricular arrhytmias. Myocarditis is a frequent cause of a arrhytmias, and is properly diagnosis by CMR. The presence of late gadolinium enhacement is relate with the diagnosis and pronostic of myocarditis.
Keywords: Ventricular extrasystole; Ventricular tachycardia; Myocarditis
BPM: Beats Per Minute; CMR: Cardiac Magnetic Resonance; LBBB: Left Bundle Branch Block; LGE: Late Gadolinium Enhacement; LV: Left Ventricule; LVOT: Left Ventricular Outflow Tract; RVOT: Right Ventricular Outflow Tract; VT: Ventricular Tachycardia; SCD: Sudden Cardiac Death
Ventricular tachycardia has a direct relationship with SCD, so risk stratification is essential for further treatment, which fluctate from expectant management or pharmacological treatment, to catheter ablation procedures or the implantation of an automatic defibrillator device. The initial step is to know the coexistence of structural heart disease using imaging technics. Myocarditis has been associated with the presence of ventricular arrhythmias with a variable prognosis. An appropriate diagnosis and risk stratification by magnetic resonance imaging can turn the treatment. The objective of the present review is to report a case of ventricular arrhythmia in which the result of CMR changed the diagnosis and subsequent treatment.
A 21 year-old man was evaluated by a cardiologist in an emergency unit with a three hours history of palpitations, lightheadedness and diaphoresis. Physical examination was unremarkable except for a heart rate of 150 bpm. No laboratory abnormalities were observed. Electrocardiogram (ECG) showed a wide QRS complex tachyarrhythmia (Figure 1) with monomorphic ventricular tachycardia (VT) criteria. After amiodarona administration, a second ECG showed sinusal rhythm and monomorphic ventricular trigeminism (LBBB pattern with inferior axis) (Figure 2). Transthoracic echocardiogram was unremarkable. In follow-up, ECG and 24 hours ambulatory Holter showed the same of monomorphic ventricular extrasystoles in 30% of the beats. In this context, the diagnosis of idiopathic ventricular tachycardia was suspected. Three weeks later, cardiac magnetic resonance was requested and basal interventricular septum myocarditis scar between both outflow tract, and systolic dysfunction (LVEF=48%) was reported (Figure 3). In that context, what is the next step?
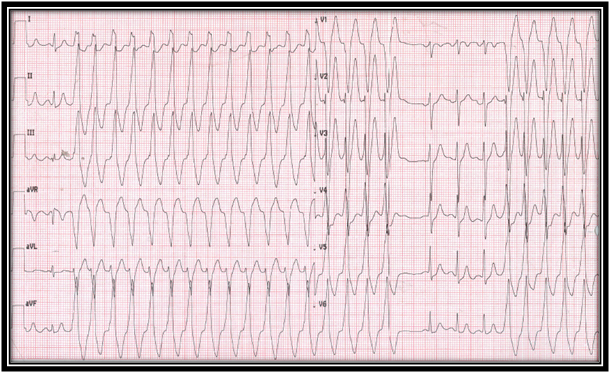
Figure 1: Widex QRS complex tachyarrhythmia. AV dissociation with AV relation ˂ 1, and onset QRS to peak in lead DII > 50 mseg was observed, supporting the diagnosis of VT.
Ventricular tachycardia is a rhythm associated with sudden cardiac death (SCD) [1], therefore an adequate diagnosis is fundamental in the prognosis. ECG is the initial tool in differential diagnosis of a wide QRS complex tachycardia. The most commonly used algorithm is the so-called Brugada algorithm or Brugada criteria, in which the isolated presence of atrioventricular dissociation has specificity of 100% to support VT diagnosis [2] (Figure 1).
Premature ventricular complexes (PVCs) are ectopic beats originated from the Purkinje system or ventricular myocardium. PVCs can be classified according to the place of origin (right or left ventricle), frequency (isolated, bigeminy, trigeminy and repetitive) shape (monomorphic, polimorphic) and relationship with cardiac cycle (relationship with diastole) [3]. In cases that PVCs or VT has a LBBB morphology with a inferior axis, idiopathic VT (adenosine-sensitive outflow tract) should be suspected4, especially in young patients without structural heart disease (Figure 2). In these cases, its important to define the possible location of the ectopic focus, most of them between or inside the right and left ventricular outflow tracts, using ECG data (Table 1 & 2) [5-8]. In our case, posterolateral superior region in RVOT is the most possible source. Evolution, treatment and prognosis of this arrhythmia could be benign [9]. In other hand, this morphology is a minor criteria of arrhythmogenic right ventricular displasia, becoming the main differential diagnosis of idiopathic VT.
QRS Morphology |
R Relationship in V1 Lead |
Precordial QRS Transition in RS |
Precordial QRS Transition in VT (da= 100%) |
V2 Transition Ratio* (da = 91%) |
V3 R/S Ratio (da=83%) |
|
RVOT |
LBBB |
Absence |
V3-V4 |
earlier than RS |
< 0.6 |
≥ 1 |
LVOT |
Present |
V1-V2 |
≥ 0.6 |
< 1 |
Table 1: Differentiation between right ventricular outflow tract versus left ventricular outflow region VT origin by 12 lead ECG.
LBBB: Left Bundle Branch Block; LVOT: Left Ventricle Outflow Tract; NSR: Normal Sinusal Rhythm; RVOT: Right Ventricle Outflow Tract; VT: Ventricular Tachycardia.
Anterior (Free wall side) versus Posterior (Septal) |
||
QRS duration (da = 80%) |
Leads II and III R wave pattern (da=86%) |
|
Free wall side |
> 140 mseg |
RR' or Rr' |
Septal |
≤ 140 mseg |
R |
Left (anteromedial attachment) versus Right (posterolateral attachemnt) |
||
Leads aVR and aVL QS wave amplitude (da = 80%) |
Lead I polarity (da = 83%) |
|
Left side |
aVR ˂ aVL |
Negative |
Right side |
aVR ≥ aVL |
Positive |
Superior versus Inferior relationship to the pulmonic valve |
||
Leads V1 and V2 initial r Wave amplitude (da = 66%) |
||
Superior (proximal below pulmonic valve) |
≥ 0.2 mV |
|
Inferior (distal below pulmonic valve) |
˂ 0.2 mV |
|
Table 2: Estimation of exactly localization of right ventricular outflow tachycardia origins by 12 lead ECG.
mseg: Milliseconds.
Mechanism of idiopathic VT in most cases are adenosine sensitive and are thought to be caused by catecholamine induced cyclic adenosine monophosphate (cAMP) mediated delayed after-depolarizations and triggered activity [10]. There are two phenotypic forms of adenosine sensitive VT, the first one is repetitive monomorphic VT (most common characterized by frequent premature ventricular complexes), and the second one is paroxysmal exercise induced VT (characterized by sustained episodes of VT precipitated by exercise or emotional stress) [11]. Idiopatic VT usually arises from outflow tracts (80-90% from RVOT), pulmonary artery, aortic cusps, papillary muscles, mitral o tricuspids inflow tracts and epicardial foci [12]. This arrhythmia appears at a relatively early age (30 - 50 years), with equal distribution between sexes in LVOT, whereas RVOT is more common in females. Common symptoms are palpitations, dizziness and syncope (rare) [13]. Acute management can be achieved by vagal maneuver or adenosine, verapamil or cardioversion; and chronic management include medical therapy (mild to moderate symptoms: beta blockers, verapamil, diltiazem, anthiarrhytmic class IA, IC and III agents). Catheter ablation is reserve for symptomatic, drug refractor patients, with a success rate of 90% and only 5% of recurrence [14].
In patients with malignant arrhythmias, CMR has been shown to be a powerful tool in the evaluation of the arrhythmogenic substrate. In a study of 82 patients with resuscitated SCD or VT, CMR diagnosed underlaying myocardial disease in 74% of cases, and 50% were reassigned to a new diagnosis [15]. In the case of myocarditis, CMR has shown a high specificity (91%) making it the gold standard now-a-days [16]. Most of these patients present a combination of oedema, hyperemia and late gadolinium enhacement (LGE), supported by the presence of systolic dysfunction and pericardial effusion. However, oedema and hyperemia are closely related to the acute event, and disappear after 3 weeks. Therefore, in the subacute or chronic phase, LGE may be the only histological mark of a recent inflammatory process. The presence of cardiac arrhythmias (PVC or VT) occur frequently in viral myocarditis. In a study involving 222 patients with biopsy proven myocarditis, 19.2% all-cause mortality was observed in the follow-up, mostly SCD-related, and LGE was the main independent predictor [17]. On the other hand, myocarditis was identified as the cause of SCD in 40% of cases in a Air Force recruits study [18]. The mechanisms by which arrhythmias occur in these patients remain controversial, however, it is hypothesized that fibrosis induced by viral inflammation leads to reentrant circuits. Other proposed mechanisms are: myocyte necrosis, proarrhythmic effects of cytokines, altered function at myocardial gap junctions, altered calcium handling, infarction and protease release [18]. In other cases, the inflammatory process could unmask an subclinical cardiomyopathy or channelopathy and predispose the genesis of malignant arrhythmias.
In most cases of malignant arrhythmias secondary to myocarditis, no further treatment is required. In general, when the inflammation turn off, the arrhythmogenic substrate also disappears, and most have a good prognosis. In cases where a scar persists, we can assume a torpid evolution with ventricular remodeling, progression to cardiac failure and a high risk of SCD. However, in the context of primary prevention, there is limited evidence to support the implantation of ICDs in patients with severe sequelae of myocarditis, so it often depends on the patient's clinical context and their own evolution.
In patients with sustained monomorphic ventricular tachycardia and frequent extrasystole it is essential to evaluate a possible anatomical substrate with imaging techniques such as CMR. Adequate etiological diagnos is related to prognosis and treatment.

©2017 Berrios-Barcenas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.