Journal of
eISSN: 2373-4396


Research Article Volume 6 Issue 3
Department of Cardiology, Zagazig University Hospital, Egypt
Correspondence: Ragab A Mahfouz, Cardiology Department, Zagazig University Hospital, Egypt
Received: November 28, 2014 | Published: August 18, 2016
Citation: Mahfouz RA, Gad M, Mohammed E, Amin (2016) Impact of Pulse Pressure Reserve on Prediabetics with Typical Chest Pain and Normal Coronary Angiography. J Cardiol Curr Res 6(3): 00208. DOI: 10.15406/jccr.2016.06.00208
Aim: To evaluate the association of reserve-pulse pressure (reserve-PP).with coronary flow reserve and diastolic function in prediabetics subjects. [Reseveve PP= Exercise pulse pressure minus rest pulse pressure).
Methods: Out of 159 patients without known CAD who were referred for coronary angiography due to typical chest pain and had normal coronary on coronary angiography, we studied 92 patients (mean age 53 ± 14years after exclusion of patients with overt diabetes mellitus. All studied patients were subjected to echo-Doppler assessment, transthoracic assessment of coronary flow reserve (CFR) and analysis of the exercise stress test.
Results: Reserve-PP was significantly lower in prediabetic compared to nondiabetic subjects (P<0.001). In addition the recovery heart rate was significantly higher in prediabetics. Prediabetic subjects had a significantly lower CFR than nondiabetic subjects (P<0.001). Reserve-PP was significantly correlated with CFR (r=0.612; P<0.0001) and inversely correlated with E/E' (r= -0.445; P<0.001). ROC analysis showed that a cut-off value of <4.5mmHg for reserve-PP had a sensitivity of 93% and specificity of 72% (AUC was 0.864; P<0.001) in prediction of impaired CFR prediabetic patients.
Conclusion: We have shown that pulse pressure reserve was blunted and associated with significant impairment in CFR and diastolic dysfunction in prediabetics. Reserve-PP might be considered a simple, non-invasive surrogate marker for subclinical atherosclerosis and diastolic dysfunction in patients with risk for coronary artery disease.
Keywords: reserve pulse pressure, normal coronary arteries, Prediabetes
Several studies reported that prediabetic subjects have unfavorable clinical outcomes.1,2 The 10years survival, only about 50% of those subjects.3 In the early phase of atherosclerosis or in the presence of intensive CAD risk factors, vasodilatation capacity of coronary resistance arterioles to pharmacological and physical stress usually disturbed before development of angiographic atherosclerotic disease.4
Tominaga et al.,5 reported that postprandial glucose level is an independent risk factor for CVD, while impaired glucose tolerance is not. Furthermore, in these patients postprandial hyperglycemia is more strongly related to the progression of atherosclerosis than is fasting hyperglycemia6 it is conceivable that pulse pressure response of the arteries to exercise, could offer critical information about vascular health. We aimed to evaluate the impact of changes in pulse pressure from resting to peak exercise (reserve pulse pressure) on coronary flow reserve and diastolic function in prediabeetics.
Out of 159 patients referred for coronary angiography for typical chest pain. We selected 92 patients (54% male) with normal coronary angiography. The mean age of them was (53.5+14years). The remaining 67 patients were excluded as they had overt diabetes mellitus (DM) Patients were classified into two groups: group 1 (included 33 subjects with a fasting plasma glucose level <5.6mmol/L); and group II prediabetic (included 59 with as a fasting glucose level ≥ 5.6mmol/L and <7.0mmol/L). Diabetic patients with a fasting plasma glucose level ≥ 7.0mmol/L or using antidiabetic medications, either oral antidiabetics or subcutaneous insulin, regardless of their plasma glucose levels.7 Exclusion criteria were: previously diagnosed cardiovascular disease, renal disease, inflammatory diseases, obesity (body mass index, BMI ≥ 30kg/m2), and/or exercise capacity. Furthermore, participants who were on regular cardioprotective medications or were acutely ill were not eligible to participate in this study. Written consent was obtained from all subjects and the study was approved from the local faculty scientific and ethical committee.
Echocardiography
With a Vivid 3 PRO ultrasound imager (General Electric, Milwaukee, WI, USA) equipped with a 2.5-5MHz (harmonics) phased-array transducer all transthoracic echocardiographic assessment was performed and left ventricular mass was calculated with the method of Devereux et al. and normalized for body surface area.10 Left ventricular diastolic function was assessed using and Tissue Doppler parameters as previously described.10
Evaluation of coronary flow reserve
Noninvasive assessment of coronary flow reserve of was performed with pulsed wave Doppler recordings of the mid-to-distal LAD. With utilizing intra-venous adenosine (0.14mg/kg/min), hyperemia was induced by the and peak diastolic velocities were measured at baseline and at hyperemia. The average the three highest peak diastolic velocities was calculated CFR equals hyperemic to baseline peak diastolic velocities ratio.11
Exercise testing
All the subjects underwent exercise stress test according to the modified Bruce protocol (Quinton Treadmill system, Quinton, Inc., Bothell, WA, USA). The formula of maximal exercise heart rate divided by (220minus age) was used to calculate the percentage of age-predicted maximum heart rate achieved during exercise. During each exercise stage and at every minute within three minutes after recovery, blood pressure, heart rate, and cardiac rhythm were recorded. Following peak exercise, the subjects were asked to walk for a 2-minute cool-down period at 1.5mph on a 2.5% grade. The maximal SBP was the highest value achieved during the test.
Blood pressure calculation
Pulse pressure (PP) = Systolic blood pressure minus diastolic blood pressure. Mean arterial pressure equals the diastolic pressure plus 1/3(PP) Pulse pressure with exercise = maximal SBP - maximal DBP. Reserve pulse pressure (Reserve-PP) = Maximum exercise-PP minus Rest-PP.
Statistical analysis
SPSS 15.0 (SPSS Science, Chicago, IL, USA) for Windows was used for statistical analysis. Univariate and multivariate regression analysis was used to test relationsstudies of the variable. Pearson's correlations were calculated to assess the correlation between reserve-PP to both CFR and E/E". Moreover, the independent relation of several variables to both E/E" and CFR in prediabetics were calculated with stepwise multiple linear regression analysis was used to test.
The baseline characteristics of the studied patients are summarized in (Table 1). among both groups, except E" and E/E" were significantly different among both groups (P<0.02 and <0.001 respectively, otherwise, all echocardiographic parameters were comparable (Table 2).
|
|
Non-Diabetics |
Prediabetics |
p-Value |
|
(n=33) |
(n=59) |
||
|
Age, (years) |
52.2±10.3 |
53.1±9.2 |
>0.05 |
|
Hypertension, n (%) |
47 (56.1) |
49 (54.0) |
>0.05 |
|
Smoking, n (%) |
38 (41.4) |
41 (46.3) |
>0.05 |
|
BMI, kg/m2 |
25.4±4.5 |
25.9±4.3 |
>0.05 |
|
Total Cholesterol (mmol/L) |
5.11±1.12 |
5.21±1.06 |
>0.05 |
|
Triglycerides ( mmol/L) |
1.54+0.85 |
1.55+0.9 |
>0.05 |
|
Glucose (mmol/L) |
4.9+0.42 |
6.0+0.45 |
<0.001 |
|
Serum Creatinine, (μmol/L) |
76.2+9.7 |
78.0+10.5 |
>0.05 |
|
SBP ( mmHg) |
129.2+10 |
133.5 +10 |
>0.05 |
|
DBP (mmHg) |
77.5+9 |
81.4+10 |
>0.05 |
|
Resting PP (mmHg) |
52.4+8.3 |
52.1+10.5 |
>0.05 |
Table 1 Demographic data of both groups
|
|
Non-Diabetics |
Prediabetics |
P Value |
|
(n=33) |
(n=59) |
||
|
LV Mass Index (g m−2) |
73.5±9.5 |
75.2±8.3 |
>0.05 |
|
Relative Wall Thickness |
0.41±0.04 |
0.43±0.05 |
>0.05 |
|
E (ms) |
0.79±0.12 |
0.69±0.11 |
>0.05 |
|
A (ms) |
0.61±0.10 |
0.71±0.14 |
>0.05 |
|
E/A |
1.16±0.19 |
0.87±0.17 |
>0.05 |
|
Isovolumic Relaxation time (ms) |
95.2±21.03 |
102.6±29 |
>0.05 |
|
Deceleration Time (ms) |
269.2±39 |
268.2±42 |
>0.05 |
|
E" (cm s) |
12.2±1.9 |
6.4±1.3 |
<0.02 |
|
A" (cm s) |
9.3±1.4 |
11.2±1.7 |
>0.05 |
|
E/E" |
6.6±1.2 |
10.7±1.3 |
<0.001 |
|
|
|
|
|
Table 2 Echocardiographic parameters of prediabetics versus controls
Maximum heart rate was significantly higher in non-diabetic compared to both prediabetics (P<0.03), while heart rate recovery was significantly higher in the prediabetics (P<0.01) (Table 3). Pulse pressure was found to be comparable among both groups at rest (P > 0.05), while it was significantly higher in nondiabetics during exercise (P < 0.001), when compared with prediabetics (Table 3).
|
|
Non-Diabetics |
Prediabetics |
P Value |
|
(n=33) |
(n=59) |
||
|
HR |
|||
|
65+9.0 |
69+9.0 |
>0.05 |
|
|
- Resting |
188+22.0 |
175+22.0 |
<0.03 |
|
- Peak |
71+11.0 |
86+15.0 |
<0.01 |
|
- HRR |
|||
|
PP |
|||
|
24.5+5.3 |
25.0+4.5 |
>0.05 |
|
|
- Resting |
35.5+4.5 |
29.2+4.6 |
<0.05 |
|
- Peak |
25.6+4.9 |
26.0+3.6 |
>0.05 |
|
- Recovery |
9.6+0.08 |
4.6+0.08 |
<0.001 |
|
- Reserve-PP |
|||
|
MAP |
|||
|
82.5+7.3 |
84.2+8.6 |
>0.05 |
|
|
- Resting |
83.2+7.0 |
87.4+8.3 |
|
|
- Peak |
82.0+6.4 |
86.5+9.1 |
|
|
- Recovery |
|
|
|
Table 3 Resting and post-exercise hemodynamic parameter among the three groups
|
|
Groups |
|
|
|
P value |
|
|
|
|
Nondiabetics |
Prediabetics |
Diabetics |
P value |
Group 1 versus group 2 |
Group 1 versus group 3 |
Group 2 versus group 3 |
|
Baseline APV |
27.2+1.5 |
25.6+1.7 |
24.8+1.8 |
<0.03 |
<0.03 |
<0.01 |
>0.05 |
|
Peak APV |
72.5±9.4 |
62.96±17.2 |
62.96±17.2 |
<0.03 |
<0.03 |
<0.001 |
>0.05 |
|
CFR |
3.23±0.29 |
1.8±0.21 |
1.8±0.21 |
<0.001 |
<0.001 |
<0.001 |
>0.05 |
Table 4 Coronary flow reserve of the three groups
With respect to CFR, the results demonstrated that it was significantly lower in prediabetics compared to nondibetics (P<0.01). Correlation analysis revealed that reserve-PP was significantly correlated with coronary flow reserve, (r=0.612; P<0.0001), and inversely correlated with E/E' ratio (r=-0.445; P<0.0001) in prediabetic patients (Figures 1 & 2). Meanwhile, PPR was significantly correlated with the heart rate recovery in prediabetcs. Regression analysis showed that reserve-PP (β=0.399, p<0.001), fasting glucose (β=-0.221, p<0.05) and waist circumference (β=-0.237, p<0.05) were independently association with impaired CFR (Table 5). The results showed that that reserve-PP (β= -0.268, p<0.001), and left ventricular mass index (β=0.198, p<0.05) have an independent association with increased E/E' (Table 6). ROC curve analysis showed that the best cut-off value of reserve-PP in prediction of impaired CFR was <4.5 mmHg with a sensitivity of 93% and a specificity of 72%. AUC was 0.864 (P<0.001), (Figure 3).
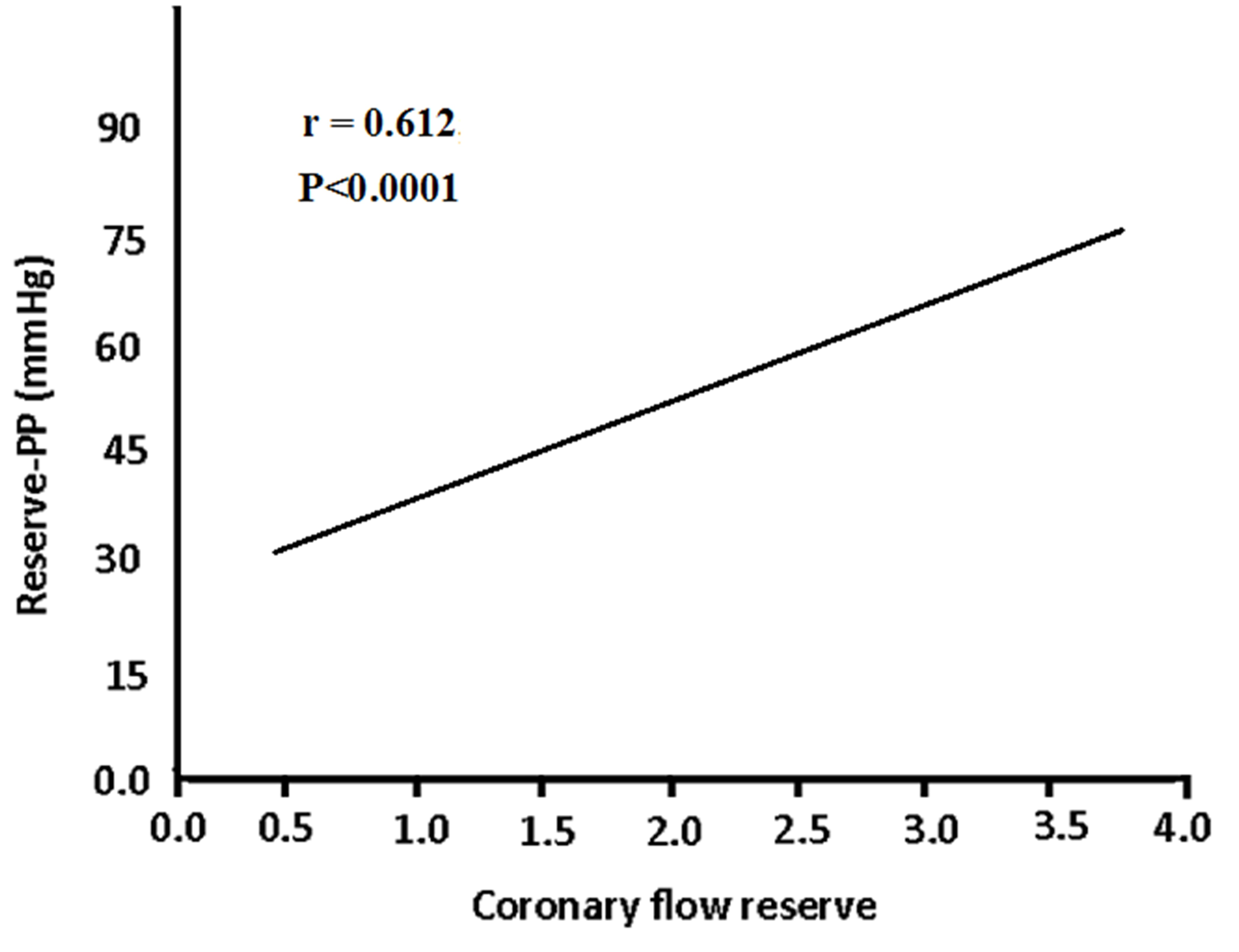
Figure 1 Correlation between reserve pulse pressure and coronary flow reserve in prediabetic patients (r = 0.612; P<0.0001).

Figure 2 Correlation between reserve pulse pressure and left ventricular diastolic function (E/E' ratio) in prediabetic patients (r = -0.445; P<0.0001).
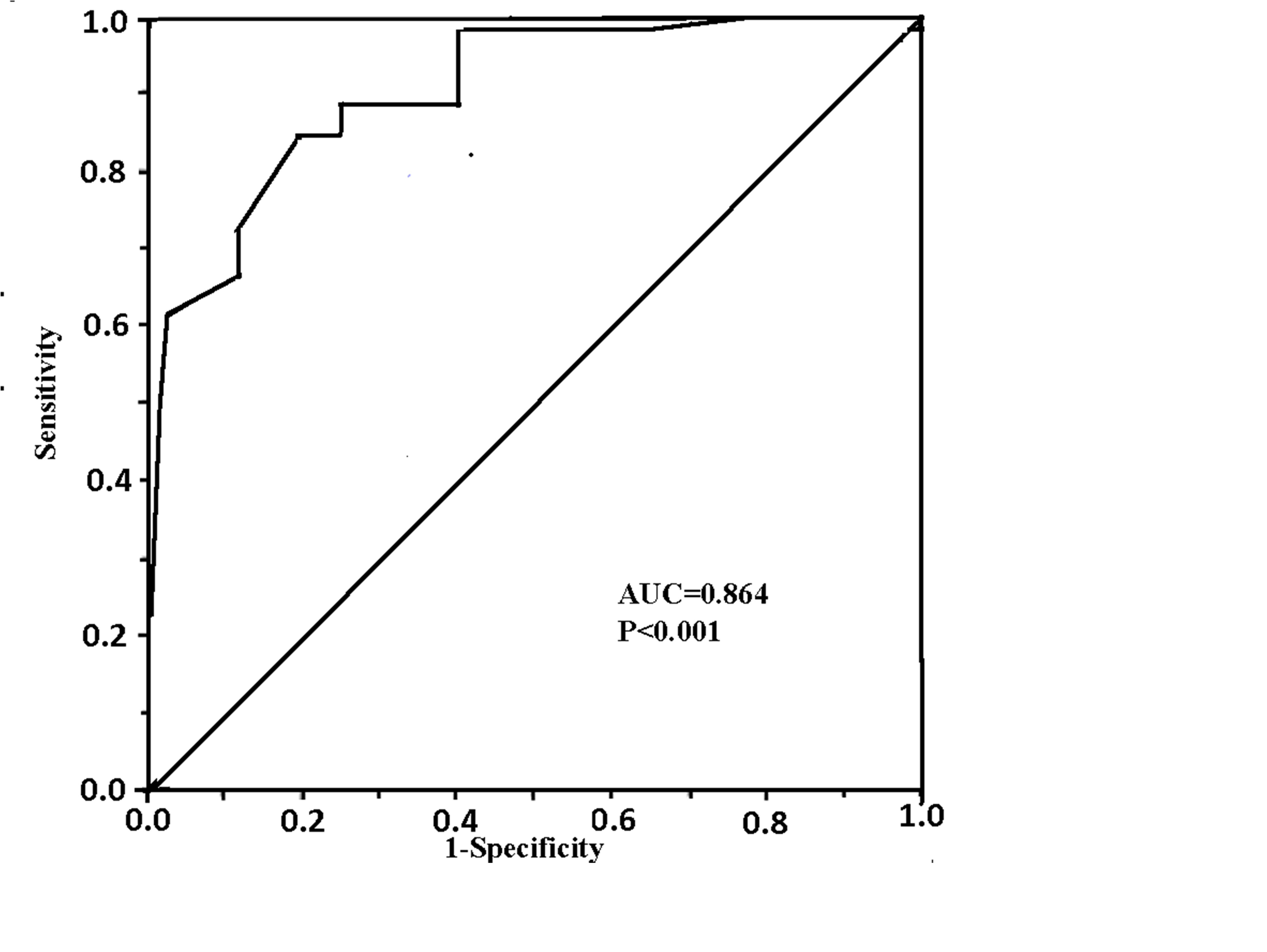
Figure 3 ROC curve analysis showed a reserve-PP of <25 mmHg had a sensitivity of 93% and a specificity of 72% in prediction of impaired CFR. AUC =0.864; P<0.001).
|
Dependent variable |
Covariate |
B Coefficient |
P value |
|
Coronary Flow Reserve |
Age |
0.114 |
>0.05 |
|
Waist circumference |
-0.237 |
<0.05 |
|
|
Systolic blood pressure |
0.109 |
>0.05 |
|
|
Diastolic blood pressure |
0.095 |
>0.05 |
|
|
Fasting glucose |
-0.221 |
<0.05 |
|
|
Triglycerides |
0.082 |
>0.05 |
|
|
LVMI |
0.122 |
>0.05 |
|
|
|
Reserve -PP |
0.399 |
<0.001 |
Table 5 Multiple linear regression analysis: Adjustment for continuous variables
LVMI: Left Ventricular Mass Index; Reverse-PP: Reserve Pulse Pressure.
|
Dependent variable |
Covariate |
B Coefficient |
P value |
|
E/E' ratio |
Age |
0.095 |
>0.05 |
|
Waist circumference |
-0.11 |
>0.05 |
|
|
Systolic blood pressure |
0.077 |
>0.05 |
|
|
Diastolic blood pressure |
0.071 |
>0.05 |
|
|
Fasting glucose |
0.102 |
>0.05 |
|
|
Triglycerides |
0.041 |
>0.05 |
|
|
LVMI |
0.198 |
<0.05 |
|
|
|
Reserve -PP |
-0.268 |
<0.001 |
Table 6 Multiple linear regression analysis: Adjustment for continuous variables
LVMI: Left Ventricular Mass Index; Reverse-PP: Reserve Pulse Pressure.
The data of our study demonstrated that the reserve pulse pressure was significantly lower in prediabetics compared to control subjects. Meanwhile the CFR and diastolic dysfunction were significantly impaired in prediabetisc. The vascular reserve was significantly correlated with CFR and left diastolic dysfunction in prediabetics.
It was observed that impaired glucose tolerance significantly associated with endothelial dysfunction and peripheral arterial dysfunction 12-14 that is commonly related to the production derived free radicals and activation of protein kinase C.15‒18 Studies showed that coronary flow reserve was significantly reduced in patients with DM even without significant CAD, usually related to coronary microcirculatory dysfunction.19,20 Reduced CFR has also been shown even in patients with prediabetes, those with impaired fasting glucose and those with glucose intolerance.21 Several studies demonstrated that vasodilatation abnormalities assessed invasively was significantly related to these metabolic abnormalities and contribute significantly on the clinical outcomes in these subjects.22,23 Microvascular dysfunction is usually caused by impaired endothelial function. In fact the endothelial dysfunction usually starts early in life. Several years before overt atherosclerosis specially in individuals with cardiovascular risk factors accompanied by insulin resistance.24‒26
The current study showed that the left ventricular function was significantly impaired in prediabetics. Meanwhile The E/E' was significantly correlated with reserve-PP in prediabetics. Moreover, CFR in prediabetics was significantly correlated with reserve pulse pressure and may be the cause of left ventricular diastolic dysfunction. Another important observation in the study was found, as the heart rate during recovery was significantly higher in prediabetics compared to nondiabetics. For now the heart rate during recovery was significantly correlated to reverse pulse pressure. Thereby blunted reserve PP could reflect an early autonomic dysfunction prediabetes.
The resting pulse pressure in healthy adults, sitting position, is about 30-40mmHg. The pulse pressure increases with exercise due to increased stroke volume, healthy values being up to pulse pressures of about 100mmHg, simultaneously as total peripheral resistance drops during exercise. In healthy individuals the pulse pressure will typically return to normal in 10minutes. Stroke volume significantly increases with increase in pulse pressure in young individuals. Pulse pressure usually increased with increasing age with middle-aged and elderly. The increase in the pulse pressures usually a marker of increased peripheral arterial rigidity. Also elevated PP associated with arterial stiffness that is correlated to higher systolic BP that could promote left ventricular hypertrophy and signify higher risk of stroke. Of note the diastolic blood pressure is concomitantly decreased, thereby compromising coronary blood flow that, in turn, might increase the risk of myocardial perfusion and diastolic dysfunction.27‒29 PP can be considered a surrogate for stroke volume.32,31 Sharman et al.,32 demonstrated that PP amplification during exercise is reduced with age and hypercholesterolemia.
The data of our study showed that PPR might be a stronger predictor of early, Microvascular dysfunction and alteration of diastolic characteristics of the heart in patients with risk factors for coronary CAD.
We found that pulse pressure reserve was blunted and associated with significant impairment in CFR and diastolic dysfunction in Prediabetics.
None.
Author declares there are no conflicts of interest.
None.

©2016 Mahfouz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.