Journal of
eISSN: 2373-4396


Review Article Volume 6 Issue 3
University Clinic of Cardiology, Medical Faculty, University ?Sts. Cyril and Methodius?, Republic of Macedonia
Correspondence: Boshev Marjan, University Clinic of Cardiology, Medical Faculty, University Campus Mother Theresa Skopje, Republic of Macedonia, Tel +389 77 54 08 08
Received: August 01, 2016 | Published: August 9, 2016
Citation: Marjan B, Magdalena O (2016) In-Stent Restenosis in Drug-Eluting Stents: Issues and Therapeutic Approach. J Cardiol Curr Res 6(3): 00206. DOI: 10.15406/jccr.2016.06.00206
Implantation of coronary drug-eluting stent (DES) became a predominant therapeutic strategy for coronary artery disease. One of the possible complications after DES implantation is in-stent restenosis (ISR) and today it becomes a significant issue for interventional cardiologists that requires further sustained and efficacious treatment. Widely accepted classification of DES-ISR is morphological classification proposed by Mehran et al. Currently available and effective therapeutic approach for DES-ISR include conventional balloon angioplasty, cutting or scoring balloon angioplasty, drug-coated/drug-eluting balloon angioplasty (DCB/DEB), DES in DES stenting (same or different), vascular brachytherapy and coronary artery bypass grafting (CABG). However, optimal treatment for DES-ISR remains unknown. Bioresorbable vascular scaffolds (BVS) offer initial hope, but further clinical studies are required to establish their long-term efficacy and safety.
Keywords:drug-eluting stents; in-stent restenosis
Introduction of coronary stents in the field of interventional cardiology has significantly improved short- and long-term results of the percutaneous coronary interventions but in the same time they become responsible for development of a new entity called neointimal hyperplasia (NIH).1 According to current available data, NIH is a process of vessel healing after stent implantation consisting of intimal proliferation as a result of local injury (barotrauma) which involves complex consecutive processes, like platelet activation and adhesion, smooth muscle cells activation, proliferation and migration to the intima, and deposition of excessive extracellular matrix.2 If overexpressed, NIH can lead to so-called in-stent restenosis (ISR), i.e. renarrowing of a previously stented vessel segment.3
In the era of bare-metal stents (BMS), restenosis rates were present in 16-44% with predomination of restenosis in longer lesions and smaller vessel diameters.1 The advent of drug-eluting stents (DES) was next logical step to eliminate the issue of NIH and ISR. DES are coated stents which release bioactive (cytostatic) agent into the surrounding areas. They were technologically invented with idea to suppress the excessive inflammatory vessel cell response to stent deployment. Unfortunately, although DES have significantly reduced restenosis rate, the entity of DES-ISR still exists and remains an issue. According to available literature reports, DES-ISR rate varies and ranges from 0-9%,2 up to 16% with first generation of DES.1
The “ideal drug” should have some properties, like anti-proliferative and anti-migratory effects on the smooth muscle cells, and should be able to promote re-endothelialization as well as inhibition of anti-inflammatory response after vessel wall injury. The “ideal stent” in order to minimize ISR must also fulfill some criteria like: to be flexible, trackable, pushable, to have low profile, to be radio-opaque, thromboresistant, biocompatible, to have high radial strength, minimal foreshortening and good circumferential coverage (optimal scaffolding) as well as to be hemodynamically compatible. The “ideal drug-delivery stent” must have larger surface area, minimal gaps and minimal strut deformation after deployment.3
Definition and classification of ISR
Generally, there are two types of restenosis described in literature. “Angiographic” restenosis means recurrent diameter stenosis (late lumen loss – LLL) >50% within the stent segment or its edges (5mm segments adjacent to the stent) in follow-up.4 “Clinical” restenosis means symptoms or ischemia recurrence with >50% diameter stenosis or >70% diameter stenosis without symptoms.5 According to widely accepted Mehran system, morphological classification of ISR includes 4 patterns (types) of restenosis: pattern I – focal (ISR ≤ 10mm), pattern II – diffuse (ISR > 10 mm), pattern 3 – proliferative (ISR > 10 mm extending outside the stent) and pattern IV – occlusion (occlusive ISR).2 This system was primarily created concerning BMS-ISR, but it also has prognostic value in DES-ISR4 (Figure 1).
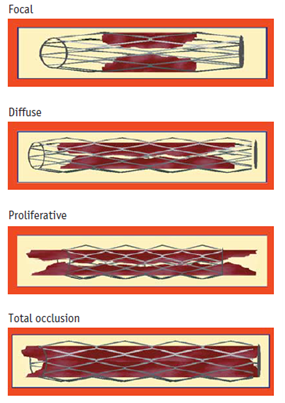
Figure 1 Classification of in-stent restenosis (ISR) in bare-metal stents according to Mehran et al.,2
In comparison to BMS-ISR, DES-ISR largely differs in terms of some important features like time of presentation, morphological characteristics, underlying substrate as well as response to further treatment.4 For example, DES-ISR most frequently exhibits focal pattern (pattern I), usually involving stent edges. In addition, patients with DES-ISR develop earlier and more frequently neoatherosclerosis than those with BMS-ISR4 (Table 1).
|
|
Bare-Metal Stent Restenosis |
Drug-Eluting Stent Restenosis |
|
Imaging features |
||
|
Angiographic morphology |
Diffuse pattern more common |
Focal pattern more common |
|
Optical coherence tomography tissue properties |
Homogeneous, high-signal band most common |
Layered structure or heterogeneous most common |
|
Time course of late luminal loss |
Late loss maximal by 6-8 months |
Ongoing late loss out to 5 years |
|
Histopathological features |
||
|
Smooth muscle cellularity |
Rich |
Hypocellular |
|
Proteoglycan content |
Moderate |
High |
|
Pen-strut fibrin and inflammation |
Occasional |
Frequent |
|
Complete endothelialization |
3-6 months |
Up to 48 months |
|
Thrombus present |
Occasional |
Occasional |
|
Neoatherosclerosis |
Relatively infrequent, late |
Relatively frequent, accelerated course |
Table 1 Comparison of Principal Features of Restenotic Tissue after Bare Metal and Drug-Eluting Stent Implantation
Etiopathogenesis of ISR
Etiopathogenetic mechanisms of DES-ISR are complex and arbitrarily can be divided into 4 main categories: biological factors, arterial factors, stent (mechanical) factors and implantation (technical) factors.1 Very often DES-ISR develops as a result of interaction of more than one factor.
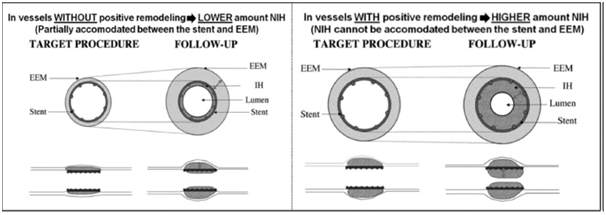
Figure 2 Explanation of the Glagov phenomenon (increased risk of ISR due to positive vessel remodeling).
Treatment options of DES-ISR
The optimal treatment for DES-ISR is not well established and remains unclear. There are available many procedures for treatment of DES-ISR with different rate of success:
In order to assess the possible mechanism for DES-ISR development as well as to serve as a guide for interventional cardiologist towards the optimal treatment, use of IVUS is highly recommended5 (Figure 4). Conventional balloon angioplasty (BA) for DES-ISR is useful treatment option which is associated with satisfactory acute results and low incidence of complications, particularly in cases with focal pattern of DES-ISR. Nevertheless, long-term results especially in cases with diffuse pattern of DES-ISR are discouraging because of the high rates of recurrent ISR. This technique is preferable in patients with clearly underexpanded stents and in those cases high-pressure balloon dilation is recommended. In addition, operator should focus only on the narrowed segment of the stent, but not the whole stented segment. One of the most frequently seen effects in such cases is so-called “dog-bone” effect which usually means shifting to use high pressure noncompliant (NC) balloons instead of compliant balloons.4 Possible complication of BA for DES-ISR is edge-related complication like edge-dissection. This can be avoided with careful and gradual balloon inflation. Commonly seen problem of BA during inflation in ISR is balloon slippage outside the stent, so-called “watermelon seeding” phenomenon, which usually occurs in cases of severe and diffuse pattern of ISR. Some authors recommend use of a buddy-wire technique to overcome this issue.4,5 However, data supporting use of BA for treatment of DES-ISR are limited, especially after the advent of DCB/DEB which becomes dominant strategy for treatment of DES-ISR.4 Cutting balloon is effective technique for treatment of DES-ISR which can prevent the issue of “watermelon seeding” phenomenon. This balloon device has tiny side blades which cut the neointima and enable balloon stabilization. Scoring balloons act on the same principle but they have superior flexibility and deliverability. Results achieved by cutting and scoring balloons are superior to those achieved by BA and they can be confirmed by some observational studies.4
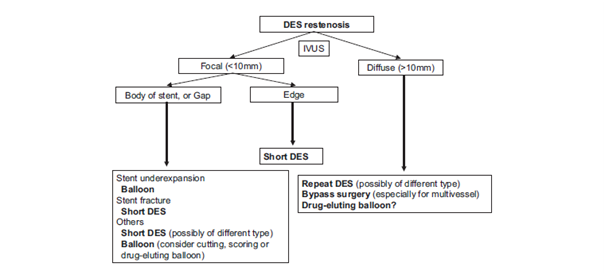
Figure 4 Algorithm for treatment of DES-ISR.5
DES: Drug-Eluting Stent; IVUS: Intravascular Ultrasound.
Drug-coated/drug-eluting balloons (DCB/DEB) have been shown to be very effective in treatment of patients with DES-ISR.4 According to a single-center, randomized study, DCB showed better clinical and angiographic results in treatment of DES-ISR in comparison to BA.9 The PEPCAD-DES study, a prospective, multicenter, randomized trial of 110 randomly assigned patients, comparing the impact of paclitaxel-coated balloon angioplasty for treatment of DES-ISR versus conventional BA showed that paclitaxel-coated balloon angioplasty was superior to BA alone for treatment of DES-ISR.10 In addition, ISAR-DESIRE 3 study, a prospective, randomized, multicenter clinical trial including 402 patients with “limus”-DES-ISR comparatively investigated the efficacy of paclitaxel-eluting balloon versus paclitaxel-eluting stent versus BA alone and confirmed that DCB/DEB was non-inferior to PES and that both DCB/DEB and PES were superior to BA alone in treatment of DES-ISR.11 Available data from these studies suggest that DCB/DEB are superior to BA in treatment of DES-ISR. Unfortunately, RIBS-IV trial demonstrated that everolimus-eluting stents (EES) provide superior angiographic and clinical results compared with DEB in patients with DES-ISR.12 Another important study, ISAR-DESIRE 4, randomizing 252 patients showed that lesion preparation with a scoring balloon before use of paclitaxel-coated balloon is linked to better outcomes than BA before paclitaxel-coated balloon in patients with limus-eluting stent-ISR.13
Available data from observational studies supports the opinion that DES offer significantly better results than other strategies including BA and cutting balloon angioplasty for treatment of DES-ISR. Whether to use DES with the same drug (homo-DES approach) or DES with different type of drug (hetero-DES approach) for treatment of DES-ISR remains an open issue and triggers wide debate in the scientific community. One of the trials in this field, the ISAR-DESIRE 2 trial which included 450 patients with sirolimus-DES-ISR has confirmed that there was no significant difference between antirestenotic efficacy and safety of sirolimus-DES (homo-DES) and paclitaxel-DES (hetero-DES) for treatment of sirolimus-DES-ISR.5,14 This conclusion does not support the theory that switch DES strategy should be used for DES-ISR. Results from this trial suggest that focal pattern of DES-ISR might not be a consequence to drug resistance as previously thought, but most probably to some other causes like gap, stent fracture, localized polymer disruption, improper drug elution or even their combination, whereas diffuse pattern of DES-ISR is probably due to drug resistance.5 Regarding bioresorbable vascular scaffolds as an option for treatment of DES-ISR, currently there are anecdotal cases only and we need data from large randomized clinical trials to confirm their efficacy and safety in these circumstances.4
There are a few observational studies that have investigated VBT for treatment of DES-ISR.15,16 In general, vascular brachytherapy is effective therapeutic approach since it provides suppression of the proliferative process and reduces rates of clinical and angiographic restenosis. Torguson et al. suggested that VBT in patients with DES-ISR was clinically useful therapeutic approach. However, use of VBT for treatment of DES-ISR has been significantly reduced in last decade since high rates of restenosis and some technical problems have been experienced.5 CABG remains the last treatment option when previously mentioned techniques have failed in attempt to solve the issue with DES-ISR. It is usually recommended for patients experiencing DES-ISR in a complex coronary lesion scenario and extended coronary artery disease (multivessel CAD) or patients with multivessel/recurrent DES-ISR.5
Despite the advent of newer generation of DES and improvement in the field of biotechnology of coronary stents, the issue of DES-ISR still exists and tends to grow due to increased use of second-generation DES worldwide. Typical morphological pattern for DES-ISR is focal and it is associated with better outcome. Incidence of diffuse pattern type is not negligible and it is usually linked to a higher incidence of recurrent DES-ISR. There are several possible treatment options for DES-ISR but the optimal treatment remains unclear. Evidences support DES and DCB/DEB as currently effective treatment approach for DES-ISR that provides acceptable clinical and angiographic results. Efforts should be made in direction to conduct large randomized clinical trials that could enlighten the mechanisms of DES-ISR and provide data on the optimal treatment approach for patients with DES-ISR. Initial hope exists with bioresorbable vascular scaffolds (BVS) but further studies are required in order to provide long-term safety and efficacy results.
None.
Author declares there are no conflicts of interest.
None.

©2016 Marjan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.