Journal of
eISSN: 2373-4396


Ablation of atrial fibrillation (AF) is our most frequently performed complex ablation procedures. Our approach to ablation of persistent AF represents a confluence of the recent literature with evolving technical capability. Pulmonary vein isolation (PVI) may be considered the cornerstone of AF ablation for any AF pattern, however recent studies identify the atrial substrate, rather than PVs, as a major source of persistent AF.1This finding is consistent with identification of rotors as drivers of human AF.2 Recognition of the potential importance of substrate and rotors was easily anticipated, given that wave break occurs constantly during AF as high-frequency wave fronts encounter structural and functional heterogeneities, with fractionation resulting in localized conduction failure and the tendency for rotation.3 It seems self-evident that certain rotors may persist, acting as drivers of AF.
We recognize that the resolution of available mapping systems continues to be the Achilles heel of rotor mapping and ablation. Nevertheless, complex fractionated atrial electrograms (CFAEs) should be found in the core region of rotors,3 although CFAEs may also arise from other processes (discharging ganglionatedplexi, heterogenous conduction, wavelet collision). In this regard, ablation of CFAEs would likely interrupt the drivers of AF, with the most dense and tenacious CFAE nests being most important to AF maintenance.4 Dr. Nademanee at White Memorial Hospital in Los Angeles demonstrates convincingly how CFAEs may be systematically mapped and ablated, with AF slowing, organizing and finally terminating. Considering the proven benefit of standard lesion sets5 and the ablation of certain CFAEs,4,6 tempered by the uncertain electrophysiologic origin of CFAEs, we have pursued a CFAE-guided approach to PVI and standard lesion sets during AF ablation. In this article, I would like to share our workflow for CFAE-guided PVI and AF ablation, which we anticipate improves outcomes.
We routinely acquire a pre-procedure CT of the LA and PV insertions. Unless patients have a CIED with monitoring ability for AF, I also strongly prefer to implant a Medtronic Reveal XT or LINQ insertable cardiac monitor at least 4 weeks prior to ablation to obtain baseline AF burden, and subsequently to monitor the success of each ablation. This serves as an invaluable guide to discontinuation of anti-arrhythmic medications, symptom correlation with any AF recurrence, and anticoagulation. We perform ablation with therapeutic INR, however prefer to hold NOACs (Xarelto, Eliquis, Pradaxa) pre-ablation, with goal to resume full dose therapy with the evening dose post ablation. We do not use Pradaxa immediately post ablation due to the lack of a reversal agent. All AF ablations are performed under general anesthesia, thereby minimizing patient motion for stable CARTO mapping. Antiarrhythmic drugs are held at least 7 days prior to ablation, with amiodarone held as long as clinically reasonable. These are resumed immediately post ablation, and continued during the 3 month blanking period post ablation.
We use four Biosense Webster catheters, including Sound star ICE, CS, Pentarray, and Surround Flow (SF) ablation catheters, with SL1 transseptal sheaths. Patients with persistent AF typically present in AF, otherwise AF is induced with burst pacing and maintained with low-dose isuprel as needed. The Pentarray catheter is first used to create RA geometry using fast activation mapping (FAM). During FAM, the CARTO software is used to perform CFAE analysis of all EGMs recorded by the Pentarray, with simultaneous generation of an atrial scar map (<0.05 mV).7 The ablation catheter is advanced within the CS to incorporate this geometry, with the Pentarray subsequently used for CFAE mapping within the CS when proximal poles of the CS catheter display complex signals. Ablation targeting the most complex signals within the RA and CS is then performed (10-20W within the CS, 20-40W within the RA, 15 cc/min irrigation) with high-output pacing (10 mV, 10 m sec) to assess for phrenic nerve capture along the lateral RA. Heparin bolus is given during RA studies, such that ACT >300 is achieved prior to transseptal puncture, minimizing the risk of thrombus formation within the arterial circulation. Finally ICE is used to create a preliminary LA geometry, localize the esophagus, and to mark the LPV carina.
As illustrated in Figure 1, the FAM of the CS and reference point at the LPV carina (Panel A, all obtained from within the RA prior to transseptal puncture) allows the CT image of the LA to be positioned precisely relative to the RA (Panel B). Following transseptal catheterization, generating the LA FAM (Panel C) is then like manipulating the Pentarray within a known geometry provided by the CT image. By preventing blind mapping of the LA, this workflow allows LA geometry to be obtained quickly and with certainty. We find the resulting geometry is often hardly distinguishable from the CT, likely due to less deformation artifact compared to traditional lasso catheters and the ability of the Pentarray splines to better align with LA contours. CT merge is therefore not needed and not performed. An added benefit of the Pentarray is less concern for entrapment in the mitral valve apparatus. Figure 2 shows CARTO displaying all LA EGMs meeting CFAE criteria (high [red dot] and medium [blue dot] confidence, default settings). We find CFAEs are typically scattered around the PV antra, with clusters found in 2-4 areas throughout the LA, which typically represents a combination of sites posterior to the LPVs, along the LA ridge, anterior to the LAA or RPVs, the interatrial septum, or along the LA floor adjacent to the CS. Comparison to the associated voltage map (not shown) indicates how CFAEs may correspond to regions of scar, serving as a further guide to effective ablation. After creation of the CFAE maps, the distribution of complex signals, as determined by the CARTO software, suggests where antral PVI (Figure 3) and linear lesion sets (LA roof, mitral isthmus, CS isolation) may be placed to maximally interrupt CFAEs. By performing such CFAE-guided AF ablation, with creation of standard lesion sets as would otherwise be indicated by clinical circumstances, patients may also benefit from CFAE ablation without increased risk for macro-reentrant tachycardias following CFAE ablation, as may sometimes occur after targeting CFAEs.6
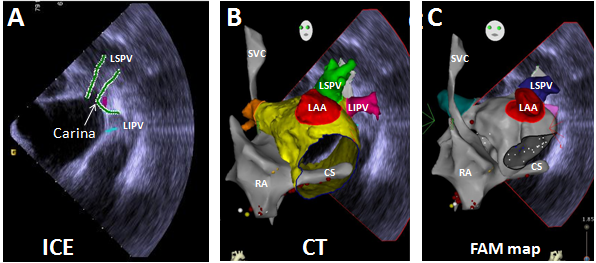
Figure 1 From the RA, ICE with CARTO Sound is used to define the LPV carina (Panel A). Using the RA and CS FAM, and LPV-carina reference point, the CT image of the LA is precisely positioned (Panel B). Creation of the LA FAM is then like manipulating the Pentarray within the CT image (Panel C), and may be performed quickly and confidently. This workflow prevents blind mapping of the LA, generating a LA geometry hardly distinguishable from the CT. CT merge is not performed.
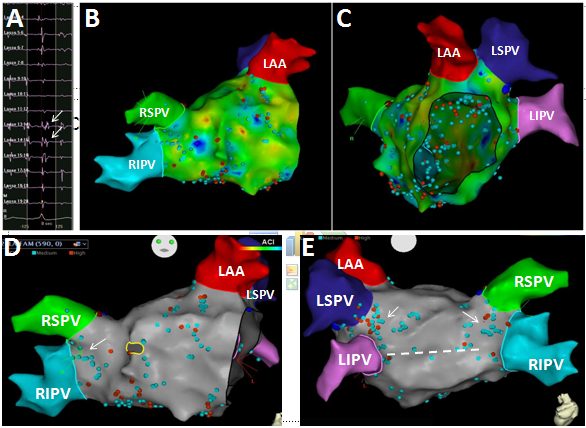
Figure 2 The pentarray catheter records a high density of local EGMs throughout the LA (Panels B & C). Signals meeting CFAE criteria (arrows, Panel A) are tagged (red, blue dots) and projected to the surface shell (Panels D & E). The distribution of CFAEs predicts where standard PVI (arrows) and linear lesion sets (dashed line) may be performed to also eliminate the most complex signals, goal to increase the efficacy of standard lesion sets.

Figure 3 PVI is performed by passing through regions of dense CFAEs until entrance and exit block is achieved for each vein. CFAEs also suggest where linear lesions may be performed along the mitral isthmus (*), or posterior LA wall (dashed line).
AF frequently terminates during CFAE-guided ablation, allowing assessment of block across ablation lines. Otherwise, ibutilide may be infused to simplify AF dynamics, allowing identification of remaining critical CFAE sites. Figure 4 shows a successful case. The patient is a 64 year-old male originally referred for pacemaker implantation and AVJ ablation for permanent AF. Instead, dofetilide was started with implantation of a Reveal XT loop recorder to monitor AF, which demonstrated a paroxysmal AF pattern in the presence of an anti-arrhythmic (Figure 4C). On this basis, AF ablation was performed October 2, 2013, with termination of AF occurring during ablation of CFAEs anterior to the LAA following CFAE-guided PVI (Panel A). Following termination, AF was completely non-inducible with atrial burst pacing, both under control conditions and following isuprel infusion. Loop recorder monitoring demonstrated no AF recurrence post ablation (Figure 4C).
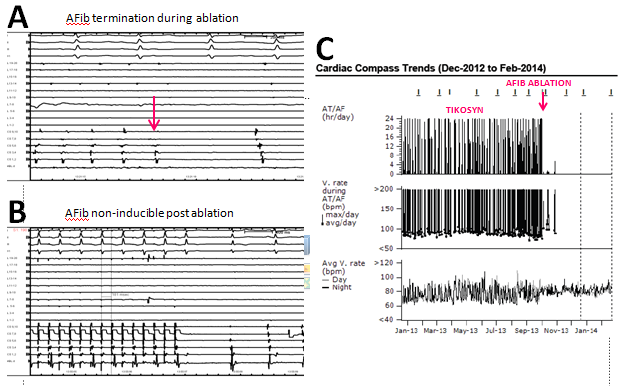
Figure 4 Termination of AF during CFAE ablation anterior to the LAA (arrow, Panel A). Following CFAE-guided PVI and AF termination, AF was non-inducible with atrial burst pacing ± isuprel (Panel B). Continuous AF monitoring with a Reveal XT loop recorder demonstrates no AF recurrence post ablation (Panel C).
In our experience, performing standard ablation lesion sets passing through regions of CFAEs is more effective than ablation performed on a purely anatomic basis. This CFAE-guided approach to PVI and linear lesions should be more effective, as it is more likely to neutralize non-PV mechanisms contributing to AF (GPs, rotors, regions of heterogeneity, atrial scar supporting focal ATs and micro-reentrant circuits). A randomized trial pursuing this hypothesis would be very interesting.
None.
The authors declare no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.