Journal of
eISSN: 2373-4396


Case Report Volume 5 Issue 6
1Radiologist, Medical Imaging Department, Saudi Arabia
2Assistant professor, King Saud Bin Abdulaziz university for health sciences KSAU-HS, Saudi Arabia
3Consultant adult congenital and structural heart disease intervention, King Faisal Cardiac Centre, Saudi Arabia
Correspondence: Saad Al Bugami, King Saud Bin Abdulaziz University, King Abdulaziz Medical City, king Faisal Cardiac center, Jeddah 21423, Saudi Arabia, Tel 966505516952
Received: May 13, 2016 | Published: May 24, 2016
Citation: Binyousef Binyousef RF, Alrahimi JS, Alkashkari WA, Althobaiti MW, Bugami SA (2016) Uncommon Findings Post Repair of Type A Aortic Dissection. J Cardiol Curr Res 5(6): 00185. RF, Alrahimi JS, Alkashkari WA, Althobaiti MW, Bugami SA (2016) Uncommon Findings Post Repair of Type A Aortic Dissection. J Cardiol Curr Res 5(6): 00185. DOI: 10.15406/jccr.2016.05.00185
Aorto-right ventricular fistulas are defects of the aortic wall in the area above the right coronary cusp, where it separates the aorta from the right ventricular outflow tract. Often, these injuries are due to trauma, infective endocarditis, rupture sinus of valsalva or occussionally following Aortic valve replacement. We describe aorto right ventricular fistula in a patient 3 months following aortic valve replacement and type A aortic dissection repair.
Keywords:aortic dissection, aorto-right ventricular fistula, aortic valve replacement
CT, computed tomography; TEE, transesophageal echocardiogram; ARV, aorto-right ventricular; RCA, right coronary artery; RV, right ventricle; TAVI, transfemoral aortic valve implanatation; RVOT, right ventricular outflow tract
A 56-year-old male patient with long standing history of essential hypertension on treatment. Sustained type A aortic dissection with severe Aortic regugitation requiring urgent surgery. He had type A aortic dissection repair. He also received a mechanical aortic valve prosthesis three months prior to presentation to our institute. He was requring follow up. He denied symptoms of chest pain, shortness of breath or palpitations. His medical therapy consisted of Asprin, Lisinopril, Atorvastatin, Metopralol and Warfarin. His biochemical profile revealed a controlled LDL cholestrol. His blood pressure and heart rate were well controlled. An ejection systolic murmur and click were auscultated over the aortic area. Electrocardiogram showed sinus rhythm and left ventricular hypertrophy. A transthoracic echocardiogram showed normal biventricular size and systolic function, well-seated mechanical aortic prosthesis with acceptable hemodynamics. An abnormal systolic flow was noted by color Doppler at the right coronary sinus directed towards the right ventricular out flow tract (Figure 1 & 2). A transesophageal echocardiogram (TEE) demonstrated a well-seated aortic prosthesis with physiological trivial intra-valvular regurgitation and a small defect measuring four millimeters between the aorta and the right ventricle with abnormal systolic flow demonstrated at the right coronary sinus directed to the right ventricular outflow tract in keeping with aorto-right ventricular (ARV) fistula (Figure 3). No echocardiographic evidence of endocarditis is seen.
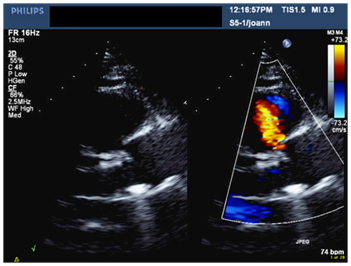
Figure 1 Parasternal long axis view in TTE showing mechanical aortic prosthesis with color flow doppler in systole indicating communication between the right coronary sinus and right ventricular outflow tract (RVOT).
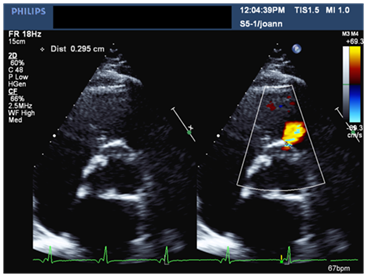
Figure 2 Parasternal short axis view at the level of the aorta in TTE demonstrating flow to the RVOT with a measured neck of ~0.3cm.
Cardiac computed tomography (CT) demonstrated patent left coronary arteries, a massive dissection flap extending from beyound the arortic graft to involve the descending aorta and a right coronary artery (RCA) dissection extending from the origin to the distal segment of RCA (Figure 4 & 5). The coronary dissection was not flow limiting and there was no evidence of obstructive coronary artery disease. The aorto-right ventricular fistula (ARV fistula) was detected by CT as a small defect four millimeters in size between the aorta and right ventricle (Figure 6).
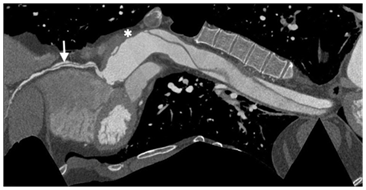
Figure 4 Curved MPR image demonstrates the dissection flap in RCA(arrow), ascending aorta graft (*) and the residual dissection flaps in the aortic arch and descending thoracic aorta.
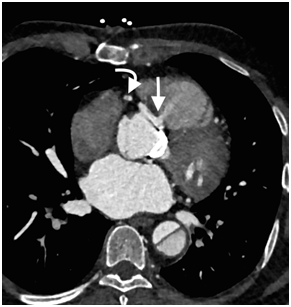
Figure 6 CT Axial image demonstrates a small defect in the aortic root and contrast jet directed from the right coronary sinus towards the right ventricle (arrow). The RCA dissection is seen in the same image (curved arrow).
Multiple factors were considered in the management plan of our patient. The percutaneous treatment of the coronary dissection deemed unnecessary as the patient was stable without signs of ongoing ischemia. A two years follow up coronary CT angiography showed the same extent of the disease with no progression.
We think the small ARV fisula is likely to be related to the surgery as there was no evidence of infective endocarditis demonstrated. Redo Surgery for this fistula deemed unnecessary and carries a grave risk of mortality and serious complication. Percutaeous closure of the ARV fistula was strongly considered but unfortunately declined by the patient. The heart team decision was to carefully follow up the patient with consideration of percutaeous closure of the ARV fistula if there is progressive increase in the size of the right ventricle (RV) or worsening of it’s function. A serial follow up echocardiographic studies after 12, 24 and 36 months intervals revealed no change in the size or function of the RV. The patient continues to do well.
Acute aortic dissection is not a very uncommon condition that could be catastrophic with a high mortality rate.1 Extension of the dissection to the coronary arteries is a rare complication, but significantly increases the mortality rate. Hirst et al. reported coronary dissection as an extension of type A aortic dissection in less than 8% of cases.2 The right coronary artery is more commonly involved.2,3 Acute coronary involvement due to aortic dissection is not always associated with a flow limiting disease. Patients with coronary involvement are usually much younger, have a higher aortic regurgitation rate and less commonly, have intramural hematoma.3
Aorto-right ventricular fistula (ARV fistula) is an exceedingly rare finding. It is mostly an acquired abnormality caused by infection, trauma or iatrogenic post surgical or percutaneous interventions. The most common iatrogenic causes for ARV fistulas are aortic valve replacement and to a lesser degree type A aortic dissection repair.4,5 Few cases have been reported post transfemoral aortic valve implanatation (TAVI).6 A well recognized rare cause of ARV fistula is rupture of sinus of valsalva aneurysms whether congenital or acquired.7 There are some exceptional reported cases of ARV fistulas related to fractured sternotomy wires and pectus bar migration.8,9
Diagnosis of aorto-right ventricular fistula was almost exclusively done by echocardiography. Confirmation of aorto-right ventricular fistula with cardiac CT was reported previously in only seven cases in English literature (Table 1),5,10˗14 four of these seven cases were congenital, two post aortic valve replacement and the last one was due to type A aortic dissection.
|
Case |
Year |
Age |
Gender |
Modality |
Cause |
Management |
Outcome |
|
Fonseca et al.,5 |
2014 |
68 |
Male |
TTE, CT |
10 years following Type A aortic dissection |
The patient was rejected for surgery due to very high surgical risk |
Sudden hypotension and death |
|
Al-Maskari et al.,10 |
2014 |
59 |
Female |
TTE, TEE, CT, Cath |
12 years following Aortic valve replacement |
Percutaneous device closure |
Doing well after 6months follow up |
|
Capin et at.,11 |
2014 |
21 |
Male |
TTE, CT |
Congenital |
Direct closure |
Not provided |
|
Masri et al.,12 |
2013 |
24 |
Male |
TTE, CT |
Congenital |
Surgical repair |
Smooth post-operative course |
|
Dwivedi et al.,13 |
2012 |
15 |
Male |
TTE, CT, Cath |
Congenital |
Surgical Closure |
Not provided |
|
Pinaud et al.,7 |
2009 |
51 |
Male |
TEE, CT, Cath |
Congenital |
Surgical Closure |
Not provided |
|
Amabile et al.,14 |
2008 |
82 |
Male |
TTE, TEE, CT |
10 years following Aortic valve replacement |
Surgical Closure |
Died |
Table 1 Cases of Aorto-right Ventricular fistula followed up by Cardiac CT
Spontaneous closure of ARV fistula has not been reported and thus, careful follow-up of all patients with ARV fistula is prudent.15,16 Treament of aorto-right ventricular fistula depends on the cause, concomitant hemodynamics status and degree of left to right shunts.
Samuels et al.,17 In their series of 40 patients with traumatic ARV fistula reported a satisfactory surgical outcome in all of the 38 patients who undergone surgical repair. The mean interval between the time of injury and definitive repair was 1.5years. Percutanous closure with septal occluders have been recently reported in patients who are poor surgical candidates.18
Imaging in patients post repair of type A aortic dissection and aortic valve replacement should search for complications of the original disease like the RCA dissection in our case that can be clinically silent and overlooked in the sitting of urgent surgery or complications related to surgery like the ARV fistula. The combination of coronary dissection and ARV fistula post type A aortic dissection repair is a very rare occurrence. The case highlights the additional values of non-invasive multimodality imaging in diagnosis, risk stratification and management planning.
None.
None.
None.

©2016 Binyousef, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.