Journal of
eISSN: 2373-4396


Mini Review Volume 5 Issue 5
Department of Cardiovascular and Thoracic Surgery and Intrathoracic Transplantation, India
Correspondence: Babar B Chaudhri, Department of Cardiovascular and Thoracic Surgery and Intrathoracic Transplantation, Sir HN Reliance Foundation Hospital, Raja Ram Mohan Roy Marg, Mumbai, 40004, India, Tel 91 7738164236
Received: December 12, 2015 | Published: May 18, 2016
Citation: Chaudhri BB (2016) SERCA 2a Gene Therapy, Game-Changer for Heart Failure. J Cardiol Curr Res 5(5): 00181. DOI: 10.15406/jccr.2016.05.00181
heart failure, calcium, SERCA
Poor uptake of Ca2+ into the sarcoplasmic reticulum (SR) is pivotal to the changes in function at the level of the cardiac myocyte in heart failure. Slow relaxation, poor contractile response to increasing stimulation frequency, accumulation of Ca2+at diastole at high stimulation rates and reduced sensitivity to βAR agonists are characteristic of cardiac myocytes from failing hearts.1˗4 Abnormal Ca2+cycling has been shown in a number of experiments, in isolated muscle strip preparations and in isolated myocytes.2
Diastolic Ca2+levels are elevated and Ca2+transients are prolonged in failing compared to non failing human myocardium. Impaired relaxation in the failing heart is due to abnormal Ca2+homeostasis.2 Intracellular Ca2+concentration (Ca2+i) transients recorded with the Ca2+probe aequorin during isometric contraction of myocardium in patients with end-stage failure were markedly prolonged, with a peak and then a secondary peak in the Ca2+i transient trace, which was associated with a marked prolongation of the time course of the Ca2+decline and of tension decline.2 Because SERCA is responsible for 60% to 90% of the Ca2+i decline in mammalian ventricular myocytes, these results were consistent with impaired function of the SR Ca2+transport system.
A critical role of SR uptake has been shown with blockade of SERCA function with either cyclopiazonic acid or thapsigargin which has been shown to blunt the force frequency response and slow relaxation to a greater extent in non-failing than failing human myocardium.5˗7
SERCA activity is reduced in the failing human myocardium.8,9 Messenger RNA levels of SERCA are reduced in the failing compared to the non failing human heart. However, at the level of protein expression, findings have been discordant. Some studies have shown a significant correlation between SERCA protein levels and myocardial function, assessed by the force frequency method. In addition, within the failing group of human hearts protein levels of SERCA differed by a factor of 4 and this variation in protein level matched differences in myocardial function.9 In a subgroup of failing hearts, therefore, SERCA protein levels are similar to those of non failing hearts and this is associated with preserved myocardial systolic function as ascertained by the force frequency relationship.
The key regulator of SERCA 2A is phospholamban (Plb), which acts as its inhibitor akin to a molecular brake. The stoichiometry of Plb to SERCA determines the level of SERCA inhibition. In the basal low phosphorylated state, inhibition of SERCA is more pronounced in the failing than non failing myocardium. Plb is a reversible inhibitor of SERCA2a, and this is relieved by phosphorylation in response to β-adrenergic stimulation. Phosphorylation is associated with increased affinity of SERCA for Ca2+and increased Vmax of Ca2+transport. Phosphorylation of Plb in situ and the accompanying increases in SR Ca2+uptake rates is partially responsible for enhanced myocardial relaxation during β-adrenergic stimulation of the heart. Plb may play the prominent role in mediation of the relaxant effects of β-adrenergic agonists because its phosphorylation and dephosphorylation correlate in time with its lusitropic effect.10 The relative Plb to SERCA ratio is thought to be critical in the regulation of myocardial contractility, therefore, alterations in this ratio may contribute to the functional deterioration in failing hearts.11 Studies of SR function in situ demonstrate a direct correlation between gene dosage of phospholamban and contractile function, and can result in a heart failure phenotype. When Plb was overexpressed in rat hearts 2.8 fold using a recombinant adenoviral vector, the animals compared to control, demonstrated lower peak left ventricular pressures, decreased peak rates of pressure rise and fall and significant increases in the time constant of left ventricular relaxation.12 In isolated myocytes there was a corresponding increase in resting cellular Ca2+, reduction of Ca2+release and prolonged phase of relaxation. These features were noted to recapitulate many of the features of heart failure. When isoprenaline was given, the maximal stimulation produced decreases in the isovolumetric relaxation time and increases in left ventricular systolic pressure similar to the control group, suggesting that in this model, the intrinsic SERCA activity was the same in Plb transfected and control group hearts, and that the phenotype seen was due to the tonic inhibition of SERCA by phospholamban.
Abnormal Ca2+regulation is primarily responsible for the slow relaxation of failing human myocardium.13 Reduced systolic force generation in the failing heart primarily results from a decreased peak systolic Ca2+level, and slowed relaxation is due to the slow decay of the Ca2+transient. Lower than normal peak systolic Ca2+of the failing myocytes results from a reduced amount of Ca2+released from the sarcoplasmic reticulum (SR), and the slower than normal rate of decay of the Ca2+transient is produced by a diminished rate of SR Ca2+uptake.14 In failing rat hearts, a mechanism for reduced SR Ca2+release appears to be abnormal coupling of trigger Ca2+(L-type Ca2+current) to SR Ca2+release. In failing human myocytes decreased SR Ca2+loading appears to be the primary explanation for decreased SR Ca2+release.14 A reduction of the SR Ca2+load in failing human heart appears to be the consequence of reduced SERCA protein; however, but this is not a universal finding.15 The consensus of studies in failing human tissues and cells is that alterations in SR function play a major role in the changes in the Ca2+transient of the failing human myocyte.15 Agents that enhance contractility in acute on chronic heart failure (HF) are limited and do not improve prognosis. Most positive inotropic agents, such as β-adrenoreceptor agonists and PDE inhibitors, cause increased mortality as a result of arrhythmia and sudden cardiac death. Figure 1 four major sites for the regulation of E-C coupling in the mammalian heart: sarcolemma, SR, Troponin/regulatory complex, and myofilaments.13
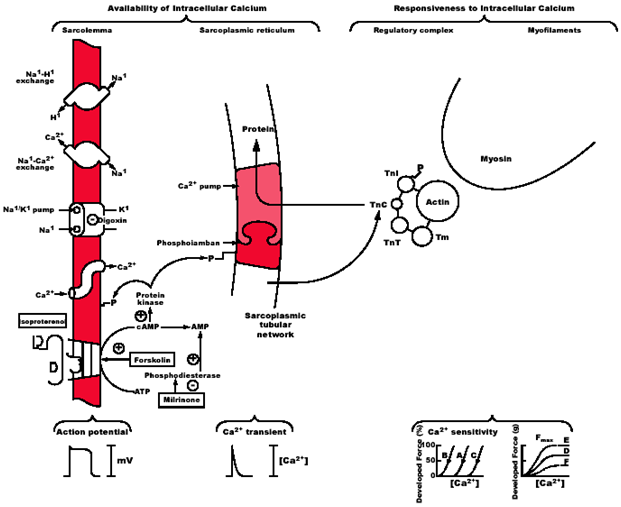
Figure 1 Four major sites for the regulation of E-C coupling in the mammalian heart: sarcolemma, SR, Troponin/regulatory complex, and myofilaments.13
Cardiac contractility may be altered by changing the availability of intracellular Ca2+for activation or the responsiveness of the myofilaments to intracellular Ca2+. Ca2+availability is regulated by predominately the sarcolemma and sarcoplasmic reticulum. Phosphorylation of L-type Ca2+channels in the sarcolemma increases their open probability. The same is true of ryanodine sensitive Ca2+channels in the sarcoplasmic reticulum. Responsiveness to intracellular Ca2+is regulated by the troponin-tropomyosin complex, and actin and myosin. Phosphorylation of troponin I reduces the apparent affinity of troponin C for Ca2+, altering the Ca2+sensitivity of the contractile elements. Phosphorylation of phospholamban blocks its inhibitory action of SERCA2, thereby stimulating ATP dependent Ca2+sequestration by the SR during relaxation. The action potential and the Ca2+transient are shown. Curves of Ca2+sensitivity and developed force are shown. Curves A and D are base line values of the sensitivities of myofilaments to calcium and the maximal Ca2+-activated force (Fmax) of fibres rendered hyper permeable to Ca2+. Ca2+ sensitivity and Fmax can change independently of one another and this is due to cAMP dependent signalling events. Curves B and E show enhancement and curves C and F show depression of Ca2+ sensitivity and Fmax respectively.13
There has been particular emphasis on calcium-transport genes as candidates for gene therapy, including SERCA2a and PLB, as well as the ryanodine receptor (RyR2), and the sodium-calcium exchanger (NCX) (Figure 1). SERCA has proven the most promising because its expression and activity are decreased in a wide variety of pathologic conditions in heart failure.12,16˗18
SERCA2a gene therapy has been tested in a wide variety of preclinical models, including acute ischaemia/reperfusion, chronic pressure overload and chronic myocardial infarction, has resulted in a reduction in ventricular arrhythmias experimental studies have demonstrated that gene therapy could be an effective option to treat the failing myocardium.18˗22
Del Monte et al.,23 first showed that over expression of SERCA2a in failing human ventricular myocytes isolated from patients with end-stage heart failure can increase SERCA pump activity and enhance contraction and relaxation velocity 17. These studies led to the development of in vivo gene transfer using catheter-based techniques to introduce SERCA2a into the myocardium.18˗23 Adenoviral mediated SERCA2a gene transfer in a rat model of pressure-overload hypertrophy (in which SERCA2a levels were decreased and severe contractile dysfunction was evident) restored both systolic and diastolic dysfunction to normal levels. Restoration of SERCA2a levels decreased left ventricular size and restored the slope of the end-diastolic pressure–dimension relationship to control levels.23 Over expression of SERCA2a in failing heart restored and normalized the levels of phosphocreatine and ATP and suggested that normalizing Ca2+ transport would improve energetics.19,23 Adenoviral mediated SERCA2a gene transfer into the infarcted myocardium significantly decreased ventricular arrhythmias, reduced infarct size, and improved wall thickening.24 An increase in Ca2+ transport and a decrease in diastolic Ca2+ and better handling of intracellular ions during reperfusion should result in improved survival of the cardiomyocytes. Improving Ca2+ transport by SERCA2a gene transfer is therefore beneficial for maintaining cardiac inotropy and for preventing the pathologic effects of Ca2+ overload.
Many of the earlier studies used adenoviral gene transfer to deliver target genes.19,21,23,24 A major disadvantage of adenoviral vectors lies in the activation of the host immune system and potential destruction of cardiac myocytes when applied in vivo. Inflammatory responses induced by adenoviral particles can be potentially fatal. New classes of vectors that provide an alternative to the adenovirus include recombinant adeno-associated virus (AAV) and lentiviral vectors. The recombinant AAV vectors can also infect nondividing cells; they are less immunogenic and do not contain viral genes, but they can accommodate only up to 4.8 kilobases of DNA. Lentiviral vectors are becoming increasingly popular because they can easily infect nonreplicating, terminally differentiated cells and can incorporate into the genome without the need for cell division may result in long-term stable gene expression in vascular smooth muscle cells and endothelial cells.
Niwano et al.,25 infused a lentiviral vector containing the SERCA2 gene into the rat heart by a hypothermic intracoronary delivery method 2 weeks after myocardial infarction (MI). The SERCA2 gene can be targeted to the myocardium using lentiviral vectors and there is improved cardiac function in a rat model of ischemic cardiomyopathy.25 This study demonstrated that the therapy prevented geometrical left ventricular remodeling after MI and also improved the survival rate. SERCA2 administration was effective even 2 weeks after an MI episode, thereby offering a potentially translatable therapy. 6 months after transduction SERCA2 gene transfer significantly prevented left ventricular dilation and improved systolic and diastolic function, resulting in reduction of mortality in the animal model used.25
As technology continues to improve, gene therapy is no longer at an experimental stage to treat heart disease. At least two clinical trials using SERCA2 gene transfer are underway: a phase I, randomized double-blinded, placebo-controlled study using AAV1-SERCA2a (Mydicar; Celladon Corporation, La Jolla, CA) in patients with congestive heart failure, and a phase I study using AAV6-SERCA2a to evaluate efficacy and safety in ischaemic cardiomyopathy patients with severe heart failure undergoing left ventricular assist device placement.18 With further development of improved delivery methods and advanced viral vectors, SERCA gene therapy may not be far from reality and could be a game changer for the management of heart failure.
None.
None.

©2016 Chaudhri. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.