Journal of
eISSN: 2373-4396


Research Article Volume 5 Issue 5
1Al-Sader Teaching hospital / Al-Najaf center for cardiac Surgery and Trans Catheter Therapy, Iraq
2Department of Pharmacology & Therapeutics, Faculty of Medicine, University of Kufa, Iraq
3Department of Pharmacology &Toxicology, Faculty of Pharmacy, University of Kufa, Iraq
Correspondence: Najah R Hadi, Department of Pharmacology & therapeutics, Faculty of Medicine, University of Kufa, Iraq
Received: April 03, 2016 | Published: May 17, 2016
Citation: Amber KI, Hadi NR, Baqir BMM, Regeeb A (2016) Impact of Coronary Novolimus Bioresorbable Scaffold & Novolimus Eluting Stent Implantation on the Acute Inflammatory Response in Diabetic Patients with Coronary Artery Disease. J Cardiol Curr Res 5(5): 00178. DOI: 10.15406/jccr.2016.05.00178
Background: The most effective treatment for coronary arteries revascularization in patients suffering from coronary artery disease (CAD) is percutaneous coronary intervention (PCI). However, when a coronary stent implantation is applied to the affected coronary artery, a mechanical injury to the arterial wall may occur causing a local inflammatory response. As a result, immediately after a PCI, a rapid increase in circulatory inflammatory markers may occur.
Objective: This study was undertaken to investigate the effect of Novolimus bioresorbable coronary scaffold and Novolimus eluting stent on the acute inflammatory response after implantation in patients with coronary artery disease.
Patients and Methods: Between January and July 2015, 44 type II diabetic patients (29 male; 15 female ) with ischemic heart disease (unstable angina) were randomly assigned into two groups: Group1: Novolimus eluting Bioresorbable coronary scaffold implantation group (20 patients) and Group 2 :Novolimus eluting coronary stent implantation group (24 patients).They were admitted to AL-Najaf center for cardiac surgery and trans catheter therapy and submitted to elective coronary stent implantation. Blood was drawn from peripheral vein directly before implantation, 12 hrs and 24 hrs after implantation and used to measure IL-8, hs-CRP, MMP-9, cTn-I and VCAM-1 levels by sandwich ELISA method.
Results: Inflammatory markers in patients subjected to elective Novolimus eluting bioresorbable coronary scaffold were found to be significantly higher than those subjected to Novolimus eluting stent. Specifically (IL-8) levels were found to be elevated at (12, 24) hrs. Levels of hs-CRP, MMP-9, VCAM-1 were found to be elevated at 24 hrs (p< 0.05) whereas the levels of cTn-I were found to be insignificantly increased (p>0.05) at (12, 24) hrs. Conclusions patients treated with BRS showed significantly higher increase in inflammatory mediators as compared to patients treated with Novolimus eluting stent.
Keywords:percutaneous coronary intervention, novolimus eluting bioresorbable coronary scaffold, novolimus eluting coronary stent; inflammatory markers
Anti HCV Ab, anti-hepatitis C virus antibody; INR, international normalized ratios; APTT, activated partial thromboplastin time; MMP-9, matrix metalloproteinase - 9; BMS, bare metal stents; PBMA, poly n-butyl methacrylate; BRS, bioresorbable scaffold; PCI, percutaneous coronary intervention; BVS, bioresorbable vascular scaffold; PLLA, poly-l-lactic acid; CAD, coronary artery disease; POBA, plain old balloon angioplasty; CTn-I, cardiac troponin-I; PT, prothrombin time; DES, drug-eluting stents; RPM, revolution per minute; ECG, electrocardiography; SEM, standard error mean; EDTA, ethylene diamine tetra acetate; Spo2, blood oxygen saturation levels; ELISA, enzyme linked immuno sorbent assay; SPSS, statistical package for the social sciences; HBSAg, hepatitis b surface antigen; ST, stent thrombosis; HIV, human immunodeficiency virus
In modern medical practice, the invasive technique of Percutaneous Coronary Intervention (PCI) is one of the most frequently performed procedures.1 Coronary stent implantation is associated with damage or intrusion of the stent into the central part of the lipid stimulates the inflammatory process in the coronary artery.2 Initially, the simple method of plain old balloon angioplasty (POBA) altered the treatment of occlusion in coronary artery disease (CAD). However, several post-POBA complications were often observed, including the re-narrowing of the artery because of elastic recoil, and sudden occlusion of the coronary artery.3 To prevent these complications, metallic stents such as bare metal stents (BMS) were introduced as a significant advancement in interventional cardiology.4 On the other hand, the long-term results post BMS implantation included occurrence of high levels of in-stent restenosis.5 In response to this problem associated with BMS, drug-eluting stents (DES) were introduced to further develop the BMS by adding the anti-proliferative drugs such as (Sirolimus or Paclitaxel). These first DES were observed to reduce in-stent restenosis when compared with the previous stent.6 DES, a substantial innovation in interventional cardiology, expanded the efficacy and safety of PCI, especially in lesions with complex properties and in patients with high risk ratios.7 But, this advancement was correlated with complications such as stent thrombosis (ST).8 Subsequent generations of DES, which were re-designed with thinner struts or biodegradable polymers, have significantly reduced these complications.9 For example, the Elixir Medical drug eluting stent is a more recently developed DES that elutes an antiproliferative drug novolimus which is a sirolimus-analogue drug. This drug provides a lower dosage when compared to different types of DES and consequently has a lower polymer load.10 While DES innovation addresses the requirements of the interventional cardiologists, it is not a best solution, as leaving a permanent cage in the vessel that can lead to further complications. Most significantly, a foreign body inside the wall of artery can cause inflammation and may impede the endothelial function, as a result delaying the healing of the vessel wall and consequently leading to increased risk of ST.11
Therefore, the best solution to overcome this problem would be a bioresorbable scaffold (BRS) that initially holds the vessel in place without causing occlusion, and then gradually dissolves. Because BRS are transient devices, they permit the vessel to return to its normal condition whilst allowing surgical revascularization in the future, if needed. In addition, a rigid permanent cage not found in the vessel wall may result in resumption of endothelial function and shear stress, decreasing the risk of delayed events and facilitating positive remodeling of the vessel.12 Despite the reduced risk of these complications, acute stent recoil in addition to the radial strength of BVS is still a concern, due to the weaker structure of the polymeric bioresorbable device compared with metallic stent.13
Since Cytokines are significant markers of inflammation, they are used as non-invasive diagnostic mediators. Interleukin 8 (IL-8) is part of the CXC chemokine subfamily.14 In unstable angina patients, neutrophils are activated immediately post procedure followed by the release of IL-8 into circulation.15 High sensitivity C-reactive protein (hs-CRP) is a sensitive marker for systemic inflammation used in predicting the risk of coronary artery disease.16 Matrix metalloproteinase (MMP)-9, defined as a neutral endopeptidase, contributes to various biological processes.17 Elevation of MMP-9 appears throughout inflammation, wound healing, and neoplasia.18 Cardiac troponin I (cTnI) is a highly sensitive and specific marker used to assess myocardial damage.19 In CAD patients, if cTn-I levels were normal pre PCI technique and were elevated post PCI, this can indicate an increased risk of future cardiac events.20 Vascular cell adhesion molecule-1 (VCAM-1) is a pro-atherogenic adhesion molecule that plays an important role in the initiation and development of atherosclerosis.21 Several research studies have revealed the role of VCAM-1 in both the inflammatory process and response to injury.22
Materials and Instruments/Materials/Novolimus eluting bioresorbable coronary scaffold system (DESolve/USA), Novolimus eluting coronary stent system (DESyne USA), Lidocaine HCL 1% (APP), Iohexol 240mg/ml (OMNIPAQUE), Heparine vial (pharmacia and Upjohn), Clot activator gel tube (Sun /Jorden), EDTA tube (AFCO/Jordan), Eppendorf tube 1.5 ml /Jordan. Human urea, creatinine, sugar, PT, PTT, INR kits (Human/Germany), Human Interleukine-8 (IL-8), high sensitive C- reactive protein (hs-CRP), Cardiac Troponin –I (cTn-I), Matrix metalloproteinases (MMP-9), Vascular cell adhesion molecule-1 (VCAM-1) enzyme linked immunosorbent assay (ELISA) kits were produced by Elabscience /china /Wuhan. Instruments / Deep freeze (-80 ̊C) (GFL/Germany), Centrifuge (K centrifuge. Tiwan), ECG monitor (City Med. China), Catheterization tools (Ireland, Germany), Bio-Elisa Reader, BioTek Instruments (USA).
Between January and July 2015, forty-four type II diabetic patients (29 male; 15 female) with ischemic heart disease (unstable angina) were admitted to Al-Najaf Center for cardiac surgery and Tran’s catheter therapy and submitted to elective coronary stent implantation. The patients were randomly assigned to two groups in a single blind study as per the following:
Group 1) includes twenty diabetic patients with coronary stenosis undergoing Novolimus Bioresorbable coronary scaffold implantation &
Group 2) includes twenty four diabetic patients with coronary stenosis undergoing Novolimus eluting stent implantation.
The following were measured one day prior to the PCI procedure: blood pressure, blood oxygen saturation
(SPO2) levels, Electrocardiography (ECG), heart rate, blood urea, serum creatinine, fasting blood sugar, and
Coagulation Factors. International normalized ratios (INR), prothrombin time (PT), activated partial thromboplastin time (APTT) were also measured. All patients were given the following viral screening tests: an anti-Hepatitis C virus antibody (Anti HCV Ab), a Hepatitis B surface antigen (HBsAg), and a Human immunodeficiency virus (HIV) test. Patient demographic information including gender, age, diabetes treatment history, and medication details were recorded for each patient.
Exclusion and inclusion criteria for study patients / for novolimus bioresorbable coronary scaffold implantation, the following patients should be excluded. Elderly patients (older than 65 years old), Non-diabetic patients, Patients undergoing long - term steroid therapy or receiving immune suppressant agents, Patients with renal impairment, Patients for whom anti-platelet or anticoagulant therapy is contraindicated, those with hypersensitivity to poly L- Lactide, Novolimus, heparin or contrast medium, as well as patients with severely calcified lesions that prevent complete balloon inflation of an angioplasty. While It should be performed in the following: Patients with myocardial ischemia (unstable angina), Patients for whom coronary artery lesion with vessel diameters between (2.75 -3.5 mm).
For Novolimus eluting coronary stent implantation, the following patients should be excluded: Non diabetic patients, Patients undergoing long-term steroid therapy or receiving immune suppressant agents, Patients with renal impairment, Patients for whom anti-platelet or anticoagulant therapy is contraindicated, those with hypersensitivity to poly n- butyl methacrylate, Novolimus, heparin or contrast medium, as well as patients with severely calcified lesion that prevent complete balloon inflation of an angioplasty. While It should be performed in the following: Patients with myocardial ischemia (unstable angina), Patients for whom coronary artery lesion with vessel diameters between (2.5-3.5 mm).
The Elixir DESolve Novolimus eluting bioresorbable coronary scaffold is comprised of bioresorbable poly L-Lactide (PLLA) based polymer and available in 3, 3.25, and 3.5 mm (diameter), and in 14, 18, and 28 mm (length) with a nominal strut thickness of 0.15 mm (150 microns). The system should be stored at (0-8) c°. While the Elixir DESyne Novolimus eluting coronary stent system is comprised of durable poly n- butyl methacrylate (PBMA) polymer and available in 2.5, 3, and 3.5 mm (diameter), and in 14, 18, 23, and 28 mm (length) with a nominal strut thickness of 0.081 mm (80 microns). The system should be stored at exactly 25C° or below.
All patients received 100 mg of aspirin and 75 mg of clopidogrel daily, beginning seven days prior to implantation and continuing for one year. During the procedure of implantation, patients received 7000-9000 units of heparine intravenously and 150-200 ml of a contrast medium Iohexol 240. The sheath was placed in a femoral or radial artery using 1% lidocaine hydrochloride as a local anaesthetic. A 6 F (0.071”) minimum guiding catheter was used with bioresorbable scaffold implantation patients; while a 5F (0.058”) minimum guiding catheter was used in cases of Novolimus eluting stent implantation. The intervention performed was determined according to standard guidelines for all patients applying routine techniques.23
Collection of samples
Blood was drawn from peripheral veins directly before implantation, as well as 12 hrs and 24 hrs after implantation, and then placed in a clot activator gel tube at 37Cº. The samples were then centrifuged at 3000 rpm at 15 mins. The samples were stored in deep freeze (-80 C°) following collection in order to measure IL-8, hs-CRP, MMP-9, cTn-I and VCAM-1 levels according to the sandwich ELISA method.
Statistical analysis
Statistical analyses were performed by using SPSS (Statistical Package for the Social Sciences), Version 20. Data were expressed as mean ± SEM. A T-test was used to compare the mean values between Group 1 & Group 2, while a Chi square test used to compare between the categorical data. In all tests P-value of less than 0.05 was designated as statistically significant.
Table 1 shows the baseline demographic characteristics, risk factors, investigations, medicate-ones of the patients who submitted to novolimus BVS and DES implantation.
|
Characteristics |
BVS Group (n=20) |
DES Group (n=24) |
P value |
|
Patients characteristics |
|||
|
Gender |
|||
|
Male |
13(65%) |
16(66.7%) |
0.908 |
|
Female |
7(35%) |
8(33.3%) |
|
|
Age (years) |
54.8±7.4 |
58.5±9.3 |
0.152 |
|
Diabetes |
20(100%) |
24(100%) |
1 |
|
Hypertension |
13(65%) |
20(83.3%) |
0.162 |
|
Systolic blood pressure (mm Hg) |
146.9±24.9 |
142.1±16 |
0.444 |
|
Diastolic blood pressure (mm Hg) |
89.7±13.6 |
87.5±11.2 |
0.567 |
|
Blood o2 saturation level (%) |
97.3±1.2 |
97.4±1.2 |
0.76 |
|
Temperature |
37.1±0.15 |
37±0.12 |
0.022 |
|
Medication |
|||
|
Statins |
19(95%) |
21(87.5%) |
0.389 |
|
Aspirin |
16(80%) |
21(87.5%) |
0.498 |
|
Clopidogrel |
18(90%) |
19(79.2%) |
0.328 |
|
B-blockers |
11(55%) |
14(58.3%) |
0.824 |
|
ACE inhibitors |
8(40%) |
11(45.8%) |
0.697 |
|
Nitrates |
8(40%) |
16(66.7%) |
0.077 |
|
Investigation |
|||
|
Fasting blood sugar (mg/dl) |
167.4±38.5 |
159.1±45.7 |
0.522 |
|
Blood Urea (mg/dl) |
29.3±6.6 |
28.8±7.3 |
0.849 |
|
Serum Creatin- ine (mg/dl) |
0.7±0.1 |
0.71±0.16 |
0.841 |
|
INR |
1.13±0.14 |
1.14±0.1 |
0.78 |
|
PT (sec.) |
13.7±1 |
14.1±0.7 |
0.215 |
|
APTT (sec.) |
27.6±1.5 |
28.4±1.4 |
0.066 |
|
ECG Characteristic |
|||
|
Heart rate (bpm) |
79.2±10 |
76.3±9 |
0.457 |
Table 1 Shows the baseline demographic characteristics, risk factors, investigations, medicate-ones of the patients who submitted to novolimus BVS and DES implantation
Effect of Novolimus bioresorbable Scaffold and Novolimus eluting stent implantation on levels of IL-8, hs-CRP, MMP-9& VCAM-1
In this study, inflammatory markers in patients subjected to elective Novolimus eluting bioresorbable coronary scaffold were found to be significantly higher. Specifically (IL-8) levels were found to be elevated at (12, 24) hrs. Levels of hs-CRP, MMP-9, VCAM-1 were found to be elevated at 24 hrs (p< 0.05) post implantation as compared with Novolimus eluting coronary stent patients, who did not experience the same increase in inflammatory markers as shown in Figure 1–4.
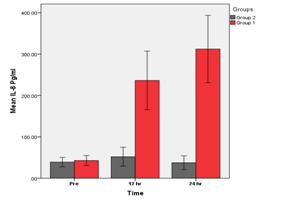
Figure 1 Groups 1 (BVS) vs. Group 2 (DES). A significant increase in IL-8 at (12, 24) hrs (p<0.05) in group 1 as compared with group 2 was found.
A Comparison of BVS &DES groups for mean of IL-8 levels at different times.
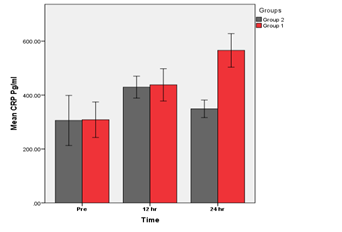
Figure 2 Groups 1 (BVS) vs. Group 2 (DES). A significant increase in hs-CRP at (24) hrs (p<0.05) in group 1 As compared with group 2 was found.
A Comparison of BVS & DES groups for mean of hs-CRP levels at different times.
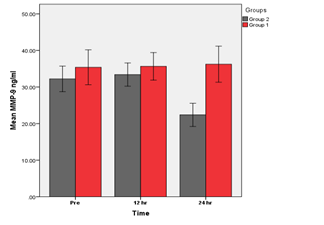
Figure 3 Groups 1 (BVS) vs. Group 2 (DES). A significant increase in MMP-9 at (24) hrs (p<0.05) in group 1 as compared with group 2 was found.
A Comparison of BVS & DES groups for mean of MMP-9 levels at different times.
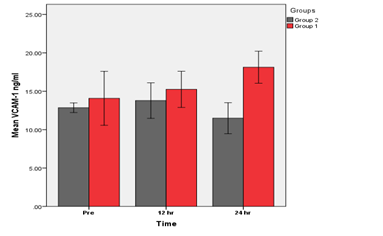
Figure 4 Groups 1 (BVS) vs. Group 2 (DES). A significant increase in hs-CRP at (24) hrs (p<0.05) in group 1 as compared with group 2 was found.
A Comparison of BVS &DES groups for mean of VCAM-1 levels at different times.
Effect of Novolimus bioresorbable Scaffold and Novolimus eluting stent implantation on level of cTn-I
This comparison of the Novolimus eluting stent and Novolimus bioresorbable scaffold implantation showed insignificant increase in levels of cTn-I at (12,24 ) hrs (p>0.05) post implantation in group 1 as compared with group 2 as shown in Figure 5.
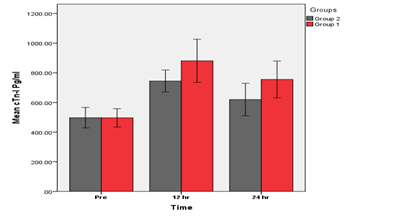
Figure 5 Groups 1 (BVS) vs. Group 2 (DES). An insignificant difference at (12, 24) hrs (p>0.05) between group 1 & group 2 was found.
A Comparison of BVS & DES groups for mean of cTn-I levels at different times There is no significant differences between the base- line of Group 1(BVS) & Group 2 (DES). (P<0.05).
Percutaneous coronary intervention is the procedure used in the treatment of coronary heart disease.24 An early inflammatory response was shown post DES implantation, and elicits after drug stent implantation systemically.25
Effect of Novolimus bioresorbable Scaffold and Novolimus eluting stent implantation on levels of IL-8, hs-CRP, MMP-9& VCAM-1
In this study, inflammatory markers in patients subjected to elective Novolimus eluting bioresorbable coronary scaffold were found to be significantly higher. Specifically (IL-8) levels were found to be elevated at (12, 24) hrs. Levels of hs-CRP, MMP-9, VCAM-1 were found to be elevated at 24 hrs (p< 0.05) post implantation as compared with Novolimus eluting coronary stent patients, who did not experience the same increase in inflammatory markers. It is common for systemic inflammatory markers to rise post PCI procedure. This elevation occurred due to stimulation in inflammatory response by injury in the vessel wall of the coronary artery, an injury incurred during balloon inflation and stent implantation.26 Caixeta et al.27 observed this effect post coronary sent implantation on IL-8 levels which increased in circulation in the early hours after PCI. Also, Moohebati et al.28,29 indicated that hs-CRP is significantly higher at 24 hrs after stent implantation. In addition, Ji et al.22 found that hs-CRP levels were increased significantly post procedure of PCI. This increase may be due to many causes including:
When compared with the novolimus eluting stent, bioresorbable scaffold implantation was found in this study to be associated with greater elevation in inflammatory markers found in DES, as described above. One possible reason for the variation between two types of coronary systems is the varying thicknesses of the struts in each device. The bioresorbable scaffold possesses a thick strut of 150 microns as compared with novolimus eluting stent with a thin strut of 80 microns and this make the device large and inflexible.32 Due to the thickness of bioresorbable struts (150 mm) and broader wall surface exposure, the thrombotic materials between device and the vessel wall might entrap more easily, as shown in DES with thin strut, therefore decreasing the squeezing of the thrombus throughout the struts.33 The bioresorbable device with a thicker strut has been shown to be more thrombogenic as compared with DES with a thin strut.34
A further possible reason for the increase in inflammatory response after BVS implantation as evidenced by higher levels of inflammatory markers in the BVS is the partial malapposition of this device, possibly due to its polymeric nature as compared with the metallic nature of the DES with properties of less radial35 and tensile strength.36 Therefore, if Absorb BVS is overstretched further than its planned limits, a fracture of the device can occur or reduction of its radial strength, because of the device’s nature.37 Although the innovation of BVS was a significant progression in interventional cardiology, there are some limitations that should be taken into consideration.
Effect of Novolimus Bioresorbable Scaffold and Novolimus Eluting Stent Implantation on Levels of cTn-I &C5a
This comparison of the Novolimus eluting stent and Novolimus bioresorbable scaffold implantation showed insignificant increase in levels of cTn-I at 12 and 24 hrs (p>0.05) post implantation in Group 1 as compared with Group 2. Increases in cTn-I level post PCI were also observed by Iliodromitis et al.38 who observed in patients with stable angina, cTn-I level increased in significant levels in blood sample at (12, 24, and 48 hrs) after PCI procedures as compared with baseline levels.
Accordingly, to the results as shown above by the increases in inflammatory mediators in patients treated with bioresorbable coronary scaffold. Large, randomized, longitudinal studies are required to examine the safety and efficacy of BVS as compared with DES and in order to know if these cardio logical innovations supersede the shortcomings and disadvantages of DES.
The bioresorbable scaffold has thick struts (150 microns), twice as thick as those of DES with thin struts. Therefore, the bioresorbable scaffolds stimulate the pro-inflammatory pathways and promote thrombus formation to a higher extent than the DES and depend on the results of this study, it can be concluded that the Novolimus bioresorbable scaffold with thick struts may because more marked inflammatory responses as compared with novolimus eluting stent with thin struts. Therefore, Patients treated with bioresorbable coronary scaffold showed significantly higher increases in inflammatory mediators at different times compared to patients treated with Novolimus eluting stent.
None.
The authors declare no conflicts of interest.
None.

©2016 Amber, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.