Journal of
eISSN: 2373-4396


Short Communication Volume 5 Issue 5
1Department of Health Sciences?s Investigation, Sanatorio Metropolitano, Fernando de la Mora, Paraguay
2Cardiology Division, First Department of Internal Medicine, Clinic Hospital, Asunci
Correspondence: Osmar Antonio Centurión, Professor of Medicine, Asuncion National University, Department of Health Sciencess Investigation, Sanatorio Metropolitano, Teniente Ettiene 215 c/ Ruta Mariscal Estigarribia,Fernando de la Mora, Paraguay, Tel 595-21-498200, Fax 595-21-205630
Received: April 26, 2016 | Published: May 3, 2016
Citation: Centurión OA (2016) Current Role of Platelet Glycoprotein IIb/IIIa Inhibition in the Therapeutic Management of Acute Coronary Syndromes in the Stent Era. J Cardiol Curr Res 5(5): 00175. DOI: 10.15406/jccr.2016.05.00175
The pathophysiology of acute coronary syndromes (ACS) is characterized by disruption of atherosclerotic plaques, activation and aggregation of platelets, and formation of an arterial thrombus. Thrombus formation can result in either transient or persistent occlusion giving rise to the spectrum of ACS. This syndrome defines rapidly evolving symptoms of myocardial ischemia ranging from unstable angina pectoris to non-Q-wave myocardial infarction to Q-wave myocardial infarction. An early pharmacological strategy to reduce ischemic complications of PCI was the use of parenteral glycoprotein (GP) IIb/IIIa inhibitors.
The benefit of GP IIb/IIIa inhibitors in early studies was driven primarily by reductions in periprocedural myocardial infarction. Advances in both stent design and adjunct pharmacology have led to improved outcomes in ACS and a diminished role for GP IIb/IIIa inhibitors as reflected in current PCI guidelines. Current medical therapy with aspirin, clopidogrel, ticagrelol, prasugrel, and heparin provides important therapeutic benefits. The platelet GP IIb/IIIa receptor antagonists, by blocking the final common pathway of platelet aggregation, are a breakthrough in the management of ACS. Several large multicenter trials have evaluated the platelet GP IIb/IIIa antagonists with and without heparin undergoing PCI or not. There was a significantly reduced incidence in the cardiac ischemic events. The clinically important benefit persisted at 3 years of follow-up. In addition to maintaining epicardial wall vessel patency, it was shown an improvement in microvascular perfusion and myocardial function as assessed by peak coronary flow velocity and regional wall motion.
Despite the success of abciximab in preventing ischemic events after PCI, the use of intravenous, small-molecule GP IIb/IIIa antagonists, and the intention to broaden the clinical indication have produced varied results. Mechanisms contributing to these heterogeneous outcomes may include the possibility of pro-thrombotic events, as well as, normal variation in platelet or receptor number, differences in receptor activity, and interpatient variation in pharmacological dose response. Trials investigating the role of intravenous small-molecule GP IIb/IIIa antagonists underline the importance of adequate higher and effective dosing. These trials highlight the use of suboptimal dose as the cause for the poor outcome in some instances. Despite the heterogeneous outcome, these agents still have potential advantages in patients with high clinical risk but low bleeding risk. Adjunctive platelet GP IIb/IIIa receptor inhibition has proved beneficial effects in the setting of PCI, and still has a place as bailout therapy for periprocedural PCI complications in ACS patients, such as those patients with large thrombus, with stent thrombosis, and refractory no-reflow phenomenon following PCI.
Keywords:platelet GP IIb/IIIa antagonists, percutaneous coronary interventions, acute coronary syndromes
Although there are major advances in the treatment and management of cardiovascular diseases, ischemic heart disease continues to be the leading cause of mortality and morbidity worldwide. Ischemic heart disease affects nearly 16 million persons aged 20 years and older in the USA. More than 2 million cases of unstable angina (UA), and nearly 4 million cases of acute myocardial infarction (AMI) occur annually in the world. These numbers will likely increase over the next decade due to aging population (1-5). The prevalence of patients who have survived an episode of AMI is estimated at 15 million, these patients are at high risk to develop additional acute events. Although, the incidence of UA is lower than that of AMI, its prevalence is likely to be higher because patients are more likely to survive an episode of UA, and these patients are also at high risk for the occurrence of additional acute coronary syndrome (ACS).
Common antiplatelet agents such as aspirin and ticlopidine, clopidogrel, ticagrelol prasugrel, and antithrombin agents such as hirudin and heparin, act by inhibiting different pathways of platelet activation in a different manner. This may account for the limited ability of these agents to prevent platelet aggregation, since other avenues of activation are left undisturbed.1-3 The critical role of the GP IIb/IIIa receptor as final common pathway for all platelet aggregation suggests the possibility that agents designed to antagonize this receptor may be the most effective type of agents for preventing platelet aggregation and, therefore, thrombosis. Regardless of the stimulus for platelet activation, GP IIb/IIIa antagonists inhibit thrombosis by preventing fibrinogen from binding to the platelet GP IIb/IIIa receptor, the final common pathway of platelet aggregation and subsequent thrombus formation.4-8 Platelets are known to play an important role in the pathogenesis of ACS. However, the high levels of platelet inhibition attainable with GP IIb/IIIa antagonists have failed to dramatically improve clinical outcomes outside of percutaneous coronary intervention (PCI). Therefore, this paper will concentrate and emphasize on the role of these anti-platelet agents in the setting where the most benefit is achieved, that is, during PCI. Although, some non-PCI trials are briefly mentioned, platelet GP IIb/IIIa studies without PCI are beyond the scope of this report. Thus, the aim of this manuscript is to review the current beneficial effects of GP IIb/IIIa inhibition in the treatment of ACS in the setting of PCI.
Platelet GP IIb/IIIa receptor inhibitors
Platelet GP IIb/IIIa receptor is distributed widely on platelet surfaces. There are an estimated 40,000 to 80,000 GP IIb/IIIa receptors per platelet, making it the most numerous receptors on the platelet membrane surface.3 Platelet aggregation is mediated by the GP IIb/IIIa receptor, a member of the integrin family of membrane-bound adhesion molecules. Integrins are defined as subunit receptors composed of a, α subunit (GP IIb), and a β subunit (GP IIIa) capable of mediated adhesive interactions between cells matrix. The two subunits of GP IIb/IIIa are encoded by separate gens on the long arm of chromosome 17. Although each subunit is synthesized separately, the receptor heterodimer must be assembled within the megakaryocyte for either subunit to be expressed.9 It was the first integrin to be identified and has served as a model for characterization of other integrin. It has been demonstrated by electron microscopy that the receptor is composed of a globular head and two flexible tails that are imbibed in the platelet membrane.10 The GP IIb/IIIa domains responsible for binding adhesive proteins have been identified and in general are characterized by their ability to recognize the peptide sequence RGD. The RGD recognition sequence was originally described for fibronectin but is now known to be present in fibrinogen, von Willebrand factor, vitronectin, and thrombospondin.11 If two activated platelets with functional GP IIb/IIIa receptors each bind the same fibrinogen molecule, a fibrinogen bridge is created between the two platelets. When these processes of aggregation are repeated thousands of times, a thrombus is generated. It is the chief receptor responsible for platelet aggregation by its ability to bind soluble fibrinogen, which forms bridges between platelets leading ultimately to thrombus formation. However, this receptor remains unable to bind fibrinogen unless the platelet is first stimulated by agonists and undergoes a conformational change. Since GP IIb/IIIa receptors are unique to platelets, and are the final common pathway for platelet aggregation by all agonists, these factors make these receptors an extremely favorable target for therapeutic pharmacologic blockade (Figure 1).12,13

Figure 1 Platelet activation pathway and site of action of antiplatelet agents.
Platelets are activated via several different membrane receptors, resulting in platelet adhesion and aggregation. When endothelium is injured, the subendothelium exposes von Willebrand factor that binds to GP Ib, causing platelet adhesion. Thrombin, TXA2, and ADP bind to the thrombin receptor, TXA2 receptor, and P2Y12, respectively. This causes an increase in intracellular calcium (Ca2+) and a decrease in cAMP, leading to platelet contraction and GP IIb/IIIa activation. Activated GP IIb/IIIa on adjacent platelets bind to fibrinogen (final common pathway) leading to platelet aggregation and thrombus formation.
Abbreviations: AA, Arachidonic Acid; COX-2, Cyclo-Oxygenase-2; CAMP, Cyclic Adenosine Monophosphate; ADP, Adenosine Diphosphate; ASA, Aspirin; UFH, Unfractionated Heparin; LMWH, low molecular weight heparin; TXA2, thromboxane A2; GP, glycoprotein; VWF, von willebrand factor; TXA2R, thromboxane A2 receptor
Reprinted with permission from Pasala T, Sattayaprasert P, Bhat PK, Athappan G, Gandhi S. Clinical and economic studies of eptifibatide in coronary stenting. Therap clin Risk Manag. 2014;10:603-614.
ABCIXIMAB (Murine monoclonal antibodies 7E3): More than three decades ago, Coller14 produced a mouse monoclonal antibody against the GP IIb/IIIa integrin receptor. The Coller antibody, termed abciximab or 7E3, has a molecular weight of 47.6 kD and exhibits both a high-affinity and absolute specificity for the GP IIb/IIIa receptor, two properties that make 7E3 an extremely attractive therapeutic agent for the blocking of adhesive proteins.15-18 Abciximab shows a high binding affinity for the GP IIb/IIIa receptor in either the active or inactive state with additional activity against the vitronectin receptor and possibly MAC-1.19,20 The vitronectin receptor plays a role in cell adhesion, migration and proliferation. The vitronectin receptor blockade can prevent smooth muscle cell hyperplasia, and MAC-1 inhibition can prevent stimulated monocyte-induced smooth muscle cell apoptosis. Abciximab has almost no renal excretion, but is cleared rapidly from the plasma, however, it is detectable bound to circulating platelets for at least 21 days.21,22
Small-Molecule Agents
Although there are several differences comparing Abciximab to the small-molecules agents (Table 1), a prospective, randomized trial comparing the platelet effects of abciximab, tirofiban and eptifibatide, demonstrated similar levels of inhibition of platelet aggregation, similar reduction in the platelet- monocyte interaction and similar mean alpha-degranulation, as a measure of antagonist-induced platelet activation.26 Although, all of these agents reduce ischemic risk to a different level, there does seem to be heterogeneity among the drugs with regard to the magnitude and durability of treatment effect, at least in the setting of PCI. Apparent variability among agents in efficacy may be due to pharmacodynamics of receptor binding, with the slow dissociation of abciximab contrasting with the rapid reversibility of other agents. Additionally, the nonspecific blockade by abciximab of both the IIb/IIIa receptor and the alphaVbeta3 receptor may theoretically provide an advantage over the specific agents. Dual receptor blockade more completely suppresses platelet-mediated thrombin generation than do inhibition of either receptor alone.
Platelet GP IIb/IIIa antagonist are potent platelet inhibitors, and thus their efficacy is greatest in conditions associated with acute platelet-mediated thrombosis. Consequently, major benefits are seen at the time of the iatrogenic arterial injury related to PCI. Attempts to expand the therapeutic indication of GP IIb/IIIa antagonists to other conditions associated with platelet-mediated thrombosis outside PCI have been less fruitful than expected.
Platelet GP IIb/IIIa inhibition in ACS during PCI
The goals of any reperfusion strategy in ACS are fast restoration of both epicardial blood flow and myocardial microcirculation. One possible mechanism by which GP IIb/IIIa antagonists exert their beneficial effects is by reducing platelet aggregation and micro-embolization downstream during mechanical reperfusion and thereby preserving the microcirculation. The role of periprocedural GP IIb/IIIa inhibition in the setting of PCI was established by several randomized, placebo-controlled trials enrolling over 30,000 patients. As adjunctive therapy for PCI, the primary objective of the randomized trials with intravenous GP IIb/IIIa inhibitors was to reduce a 30-day ischemic composite end point, namely, death, myocardial infarction (MI), urgent revascularization.The most compelling support for platelet GP IIb/IIIa inhibition therapy comes from the abciximab trials. They have demonstrated a clinically important reduction in early ischemic events, sustained beneficial effects at long-term follow-up, and benefits that extends similarly to all interventional devices, lesion complexities and patient acuities.
Pioneering Clinical Trials
The EPIC,27 EPILOG,28 and EPISTENT29 trials demonstrated the efficacy of abciximab in reducing complications among patients at high risk during PCI. There was a significantly higher reduction in the 30-day ischemic composite end point of 35% (p=0.008), 56% (p<0.001) and 51% (p<0.001) respectively. The greatest magnitude of clinical benefit with this agent is achieved in patients undergoing PCI, wherein the timing of plaque injury and the role of acute platelet aggregation are precisely defined. There are up to 65 acute ischemic events prevented per 1000 patients treated. Inhibition of acute ischemic events is achieved primarily in the first 12 to 48 hs after revascularization and maintained over three year follow-up. However, important questions were raised regarding whether such a strategy should be used for all patients undergoing PCI, the timing of the revascularization procedure and the optimal duration of the post-procedural drug infusion. In this regard, the CAPTURE30 trial reported a relative risk reduction of only 23% (p=0.012). Although it was a significant reduction, it was far lesser than the relative risk reduction obtained with the EPI-trials. This difference in the results may be related to the different duration of the post-procedural drug infusion. Although, the CAPTURE trial had a long duration of the pre-procedural drug infusion (18 to 24 h), it had only a 1 h post-procedural infusion. In comparison, the EPI-trials employed a 12 h post-procedural drug infusion. Thus, the CAPTURE patient’s platelets were more likely to be under 80% inhibited, and also returned to normal function sooner after PCI.
Similar positive results with lesser statistical significance were obtained with the small-molecule agents. The IMPACT II and RESTORE trials provided evidence that eptifibatide and tirofiban, respectively, also diminished periprocedural ischemic events, but the magnitude of treatment effect with this agents was less than in the abciximab trials and did not reach conventional levels of statistical significance.31-33 The same holds true for lamifiban which was studied in a randomized, placebo-controlled manner in the PARAGON B clinical trial.34,35 Ischemic events were reduced considerably by eptifibatide or tirofiban at 24 to 48 hrs, but attenuation of clinical benefit occurred over the subsequent 30 days. The composite end point of death, MI, or urgent revascularization at 30 days for eptifibatide compared to placebo was (9.9% vs 11.4%, p=0.220), and for tirofiban was (8.0% vs 10.5%, p=0.052). Higher dosing regimens had improved the clinical outcome with the small molecule antagonists. The interaction of platelet glycoprotein IIb/IIIa blockade and coronary revascularization was not tested in a randomized fashion in the PURSUIT and PRISM PLUS trials that utilized eptifibatide and tirofiban, respectively.36,37 However, a substantial number of patients underwent PCI during study drug infusion, although selection for this procedure was a post-randomization event and may have been influenced by the occurrence of ischemic end points. Nevertheless, the consistent finding in these two trials was that GP IIb/IIIa blockade was effective in stabilizing patients before the performance of PCI and in reducing ischemic events after mechanical revascularization. In the PURSUIT trial, 1228 patients underwent PCI within the first 72 hs. Eptifibatide therapy in these patients was associated with a reduction in the composite end point of death or MI by 30 days (16.7% vs 11.6%, p=0.01, absolute risk reduction of 5.1%), and a significant reduction in the risk of MI before mechanical revascularization (5.5% vs 1.7%, p<0.001). The relatively modest treatment effect with eptifibatide in IMPACT II was almost certainly due to inadequate dosing. The potential for higher doses of eptifibatide to more effectively reduce ischemic complications is suggested by the substantial treatment effect among patients in PURSUIT trial who underwent early PCI. In the PRISM PLUS trial, angioplasty was performed in only 30.5% of patients. The patients who underwent PCI appeared to derive particular benefit from pretreatment with tirofiban plus heparin. Though the analysis of patients who underwent PCI is not based on a randomized cohort, patients treated with tirofiban had a 46% reduction in cardiac ischemic events after PCI, including a 43% reduction in the composite end point of death or MI. Likewise, a 43% (p=0.0017) reduction in the same end point at 48 hs was reported in the ESPRIT trial in which 98% of the enrolled patients underwent PCI with a 96% rate of stent implantation.38 A sub-study of this trial has shown that in the modern era of PCI and in an extremely homogeneous population, the GP IIb/IIIa receptor blockade with eptifibatide is an effective therapy to prevent ischemic complications similarly in women and in men.39 The TACTICS-TIMI 18 trial (n=1,851) had confirmed the benefit of an invasive approach in patients with ACS treated with tirofiban, particularly in patients with a positive troponin with an absolute reduction in the primary composite end point of death, MI, and re-hospitalization for ACS at six month of 10%. Among patients with MI, those with troponin elevation before the initiation of reperfusion therapy are at high risk for death and heart failure. In this setting, the prognostic value of troponin and serum myoglobin is independent of baseline variables, time to initiation of PCI, infarct location, and measures of the efficacy of reperfusion therapy.40-42
In the EPIC trial, abciximab infusion was associated with a reduction in target vessel revascularization procedures at 6 months, from 22% to 16% (p=0.007), a finding that led to the speculation that this drug may reduce restenosis.43 This significant decrease in the need for late target vessel revascularization was not observed in the subsequent trials of EPILOG and EPISTENT. Furthermore, the ERASER trial, using intravascular ultrasound examination, did not find a reduction of in-stent restenosis in De novo native coronary lesion in patients undergoing stent implantation with adjunctive abciximab.44 However, the EPISTENT trial demonstrated that in a subgrup of patients with diabetes mellitus there was a 51% (p=0.002) restenosis reduction.45 The processes of recoil and remodeling are not operative with stents and neointimal hyperplasia is the predominant mechanism of luminal re-narrowing.46 Thus, in the setting of stenting, abciximab may reduce restenosis in diabetic patients, who are known to be at high risk for this complication.
At the time when trials of GP IIb/IIIa inhibitors were assessing balloon angioplasty and atherectomy, there was a substantial change in the approach of coronary stenotic lesions. The therapeutic practice turned into the direction of stenting. Two large randomized international trials demonstrated the unquestionable superiority of stents over balloon angioplasty, mainly because of significantly less incidence of restenosis and reduced necessity for repeat revascularization.47,48 Stenting activates expression of the GP IIb/IIIa receptor on the platelet surface and predisposes to coronary thrombus, which is partly reflected by the clinical syndrome known as subacute thrombosis.49 The use of a metal prosthetic device during the procedure may also lead to mural thrombus inside the stent, distal emboli of atherosclerotic material or fragmented thrombus, and predispose to deeper arterial wall trauma and platelet activation because of high pressure inflation. These pathophysiological triggers may be more adequately and effectively controlled with the association of platelet GP IIb/IIIa receptor antagonists to stenting. The GP IIb/IIIa receptors antagonists have shown that can maintain patency of the re-canalized vessel, besides avoiding emboli of platelet aggregates to the distal circulation.50
The EPISTENT trial showed that stenting associated to inhibition of the final common pathway of platelet aggregation by GP IIb/IIIa antagonists is superior to stenting alone. If the EPISTENT results are applied to all PCI procedures worldwide, the use of abciximab would be expected to prevent more than 2,500 deaths, 40,000 MI, and more than 6,500 emergency revascularization procedures.29 The CADILLAC trial has also shown that abciximab further improves early and late clinical outcomes in patients treated with stent implantation, but especially in patients undergoing balloon PCI alone.51 The reduction in the 30-day MACE rate with abciximab was more pronounced in patients assigned to balloon angioplasty (4.3% vs 8.1%, RR 47%, p=0.01) than in those assigned to stenting (4.2% vs 5.3%, RR 21%, NS). Therefore, these findings highlight the beneficial effects of platelet GP IIb/IIIa receptor blockade in PCI, which is more pronounced in patients undergoing balloon angioplasty, probably due to the less internal luminal diameter achieved with this procedure compared to stenting. A meta-analysis of 3 randomized trials (ISAR II, ADMIRAL, CADILLAC) on stent placement in AMI with either adjunctive abciximab or placebo was described.52 Adjunctive use of abciximab in stent treated patients resulted in an event rate of 12% vs 16.6% without abciximab (OR 0.70; 95% CI 0.54-0.92, p<0.001). Thus, abciximab prevented 44 major events per 1000 patients treated. It was concluded that optimal mechanical reperfusion in AMI is provided by stent placement and GP IIb/IIIa use, with a 30% odd reduction in the composite end point. It was demonstrated that even in very high risk patients in cardiogenic shock there is a beneficial effect. Death was reduced by 57% with abciximab compared to patients with placebo.53 Two observational, non-randomized studies have shown that treatment with platelet GP IIb/IIIa inhibitor abciximab during angioplasty resulted in improved outcome of patients with shock complicating AMI.54,55 Similar results were obtained with eptifibatide in this subgroup of high risk patients. Although, only 25% of the shock-patients underwent PCI, a retrospective sub-study of the PURSUIT trial demonstrated that patients treated with eptifibatide seemed to have reduced adjusted odds of 30-day death from shock (OR=0.51, 95% CI 0.28-0.94; p=0.03), suggesting a possible salutary effect of platelet GP II/b/IIIa blockade during shock.56 Since this finding is derived from a post hoc analysis, it should also be verified in specifically designed studies.
Several trials compared the relative efficacy of two agents in patients undergoing coronary stenting.57-59 In the TARGET trial, patients were randomized in a double-blind fashion to tirofiban (n=2,398) or abciximab (n=2414) in addition to aspirin, heparin and clopidogrel.57 The primary end point was 30-day death, MI, and urgent revascularization, and the trial was powered to demonstrate the non-inferiority of tirofiban. Abciximab, compared to tirofiban, produced an absolute reduction in the primary end point of 1.54% (6.01% vs 7.55%, p=0.037). The benefit of abciximab appeared early, reducing MI within 12 hs of the bolus. The authors concluded that, although the trial was intended to assess the noninferiority of tirofiban as compared with abciximab, the findings demonstrated that tirofiban offered less protection from major ischemic events than did abciximab (Figure 2). Similar conclusions can be obtained from the AU-Assessing Ultegra (GOLD) study, which related the degree of inhibition after an abciximab bolus and 12 h infusion to ischemic outcome in patients undergoing PCI.60 However, it is very important to emphasize that in the absence of intervention, the benefit is of lesser magnitude and for abciximab in particular, as seen in the GUSTO IV trial, it may be reversed or associated with paradoxical harm.61 There was a stepwise increase in events from 8.0% in the placebo group to 9.1% in the 48 h treated group. This represents a 14% increase in death or MI primary end point for the long-infusion abciximab strategy. This may be due to paradoxical platelet activation when there is less than 80% platelet inhibition. Platelet inhibition of >80% is required for maximum efficacy. Failure to achieve these high levels of inhibition is associated with lack of protection from acute events. Moderate levels of platelet inhibition not only lack efficacy but are associated with a paradoxical increase in ischemic complications due to the unmasking antagonist-induced pro-thrombotic and pro-inflammatory effects.62 During an abciximab infusion, more than 25% of patients are less than 80% inhibited increasing the possibility of important thrombotic, counter regulatory events. Thus, abciximab should not be used in unstable coronary syndromes in the absence of PCI. The TARGET trial confirms the superiority of abciximab over tirofiban in the setting of PCI.57
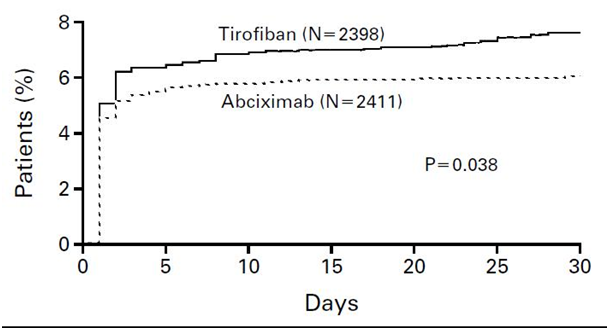
Figure 2 Incidence of the primary end point in the target trial.
Incidence of the Primary End Point, a Composite of Death, Nonfatal Myocardial Infarction, or Urgent Target-Vessel Revascularization, in the First 30 Days after Enrollment. After 30 days, the incidence of the primary end point was 7.6 percent in the tirofiban group and 6.0 percent in the abciximab group (hazard ratio, 1.26; 95 percent confidence interval, 1.01 to 1.57; P=0.038).
Reprinted with permission from Topol EJ, Moliterno DJ, Hermann HC, et al. For the TARGET Trial investigators. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for thr prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344:1888-1894.
On the other hand, depending on the time of initiation of the pharmacological agent, the outcome may vary accordingly. The EARLY ACS trial investigated the same drug administered in two different periods of time during the course of the ACS.58 The authors observed in patients who had ACS without ST-segment elevation, that the use of eptifibatide 12 h or more before angiography was not superior to the provisional use of eptifibatide after angiography (Figure 3). The early use of eptifibatide was associated with an increased risk of non–life-threatening bleeding and need for transfusion. The duration of the infusion time was also investigated in the BRIEF PCI trial.59 The investigators demonstrated that after uncomplicated PCI, eptifibatide infusion can be abbreviated safely to less than 2 h. It is not inferior to the standard 18-h infusion in preventing ischemic outcome, and it may be associated with less major bleeding.
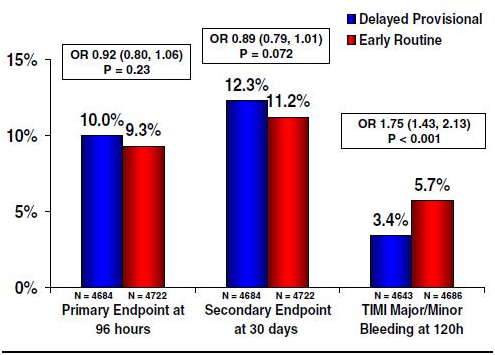
Figure 3 Major efficacy and safety. results in the EARLY ACS trial.
Rates of the primary efficacy composite end point (death, myocardial infarction, recurrent ischemia leading to urgent revascularization, or thrombotic bailout) at 96 h, key secondary efficacy composite (death or myocardial infarction) at 30 days, and the primary safety end point (TIMI major bleeding) are shown for a delayed, provisional use of eptifibatide just prior to percutaneous coronary intervention (blue) versus the routine early administration of eptifibatide (red). OR: odds ratio.
Reprinted with permission from Giugliano RP, Braunwald E. The year in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2009;54(16):1544-1555.
Several trials demonstrated that adding Platelet GP IIb/IIIa receptor inhibition therapy to PCI resulted in several beneficial effects.63 It was demonstrated that 1) has both short-and long-term reduction in ischemic complications, 2) improves microcirculatory function and recovery of myocardial function, 3) prevents the development of restenosis in diabetic patients and 4) reduces long-term mortality. Proposed mechanisms to explain this benefit include inhibition of platelet aggregation, reduced platelet-mediated thrombin generation, reduced release from platelets of vasoactive agents, reduced platelet-mediated clot retraction and reduced intimal hyperplasia in response to vascular injury.64-68 In a well done experimental study of stenting and abciximab, it was demonstrated that abciximab treatment reduced platelet thrombus formation area by 89%, but did not prevent the deposition of a discontinuous monolayer of platelet, demonstrating that abciximab blocks platelet to platelet interactions, but not platelet adhesion.69 The authors of this experimental study proposed that stent insertion leads to a reduction in blood flow immediately adjacent to the wall, which favors thrombin generation and the development of a fibrin network that can trap the nearby layer of stagnant blood. The most plausible explanation for abciximab’s effect is that it decreases platelet thrombus formation at the sites where the stent struts contact the vessel wall and thus decreases thrombin generation and fibrin formation. There were also significantly smaller platelet thrombi at the site of stent insertion which explains the protective effect of abciximab on the microcirculation.69 A study designed to assess endothelium-dependent vasomotion after coronary stenting plus abciximab demonstrated that abciximab preserves the coronary blood flow response to acetylcholine after coronary stenting. Acetylcholine-mediated increase in coronary blood flow was impaired after stenting, however when it was associated to abciximab there was a significantly superior response than in the stent alone group. The preservation of microvascular endothelial function may help explain the beneficial clinical effects of GP IIb/IIIa receptor antagonists in patients undergoing PCI.70
There are several factors to consider in the safety profile of the platelet GP IIb/IIIa inhibitors. The reduction of bleeding complications in the PCI plus GP IIb/IIIa antagonists trials demonstrates that reduction of concomitant heparin doses, as well as early vascular sheath removal ameliorate excess hemorrhagic risk in this setting. Thrombocytopenia occurs infrequently with GP IIb/IIIa inhibition but may be precipitous and profound (platelet count of <50,000 mm3). It occurred in 7% of patients randomized in the PURSUIT trial and it was an independent predictor of 30 day death and MI. Therefore, platelet count should be measured early (within the first 2 to 4 hs) after administering these agents and followed for the duration of therapy. Pseudo-thrombocytopenia is the cause of more than one third (36%) of low platelet counts in patients undergoing PCI who are treated with abciximab. Pseudo-thrombocytopenia is a benign laboratory condition that does not increase the risk of bleeding, stroke, transfusion requirements or the need for repeat revascularization. Besides, it does not require specific therapy. Therefore, it is important to recognize this condition so that the beneficial effects of abciximab are not lost by premature termination of therapy.71 Due to its murine origin, abciximab elicits an immune response in the form of IgG antibody production, termed the human anti-chimeric antibody (HACA), in 7% of patients within the first month after initiation of therapy. This does not occur with the small molecule antagonists. Abciximab re-administration in this situation is safe and does not result in an allergic response or any increase in adverse events, although, increased levels of thrombocytopenia are seen. The presence or absence of a positive HACA titer was not predictive of a lack of clinical effectiveness, development of thrombocytopenia, or other sequelae in patients undergoing re-administration. Thus, overall side effects are similar with the different agents and usually not a major clinical problem.
Lessons from meta-analysis and recent studies
Boersma E et al.,72 found that treatment with GP IIb/IIIa inhibitors led to a 9% reduction in the RR of death or MI with a concurrent 1% absolute increase in major bleeding in a meta-analysis of more than 30,000 patients with ACS. However, only 24% of these patients underwent PCI. More recently, Sethi A et al.,73 performed a meta-analysis of randomized trials of GP IIb/IIIa inhibitors use in patients undergoing primary PCI. In this study of more than 7,000 patients, GP IIb/IIIa inhibitors use was associated with a 25% reduction in mortality. Meta-regression analysis suggested that the benefits of GP IIb/IIIa inhibitors use were confined to patients at highest risk of mortality. Moreover, Winchester DE et al.,74 performed a meta-analysis of GP IIb/IIIa inhibitors use in ACS and PCI on the basis of trials performed in the contemporary era of stents and dual antiplatelet therapy. Among ACS patients, GP IIb/IIIa inhibitors use was associated with a significant reduction in nonfatal MI and an increase in minor bleeding but no differences in mortality or major bleeding. Thus, although there are numerous differences in patient populations, timing of drug administration, and concomitant medical therapy, most studies have tended to demonstrate that GP IIb/IIIa inhibitors use in ACS and PCI leads to modest benefits in terms of ischemic complications with a concomitant increase in bleeding, particularly in the highest risk patients.
Safley DM et al.,75 utilized the National Cardiovascular Data Registry, CathPCI Registry data to investigate the association between GP IIb/IIIa inhibitors use and PCI outcomes for ACS. The primary outcome was all-cause in-hospital mortality. The secondary outcome was major bleeding. In their study of nearly one million patients undergoing PCI for ACS, they found that GP IIb/IIIa inhibitors use was associated with lower mortality but higher rates of major bleeding. These results were similar regardless of the analytic approach used to adjust for potential differences between those patients who did and did not receive these agents. Subgroup analysis demonstrated that the mortality benefit of GP IIb/IIIa inhibitors in this setting was restricted to patients with ST-segment elevation MI and those treated with heparin anticoagulation. There was no evidence of differential benefit or harm according to the radial or femoral access site. Since the patients who received GP IIb/IIIa inhibitors tended to be sicker and more complex, their use was associated with higher mortality in unadjusted analysis. However, in adjusted analyses the use of GP IIb/IIIa inhibitors was associated with reduced mortality.75
Facilitated PCI
The term facilitated PCI refers to early, planned PCI after the administration of a pharmacological regimen intended to open the infarct-related artery. Gibson CM described four components to the time-dependent “open vasculature” hypothesis. The achievement of 1) early flow, 2) full microvascular flow, 3) full epicardial flow, and 4) sustained flow, are all related to improved clinical outcomes in AMI.76 The platelet GP IIb/IIIa receptor blockade and reduced-dose thrombolytics may more fully satisfy the first two criteria. They rapidly open epicardial vessels and the microvasculature. On the other hand, mechanical interventions may better satisfy the latter two criteria, offering full and sustained reperfusion. Clinical outcomes are related not merely to the attainment of one, but rather to the fulfillment of all four prerequisites of the open vasculature hypothesis. It would therefore be logical to offer the best of the two complimentary strategies by combining the speed of patency and improved microvascular function provided by a pharmacologic strategy with the speed of flow following more definitive mechanical intervention. It is possible that early PCI after pharmacologic reperfusion therapy has the potential to fuse the best aspects of fibrinolysis, antiplatelet drugs and mechanical reperfusion. Combination therapy also allows the use of lower doses of thrombolytic agents, which may in turn potentially reduce the risk of intracranial hemorrhage; and it combats the pro-thrombotic state induced by thrombolytic agents. Thus, Platelet GP IIb/IIIa receptor antagonists may be well suited as an adjunct to both thrombolytic and mechanical interventions in AMI. Another advantage of facilitated early PCI relates to patient stability in the catheterization laboratory. Patients arriving in the laboratory with patent infarct-related artery due to earlier reperfusion with reduced-dose thrombolytic and GP IIb/IIIa antagonists are less likely to be in cardiogenic shock.77 These are plausible theoretical assumptions that were not fully demonstrated.
Early trials of rescue PCI did not include, for the most part, the use of either stents or adjunctive GP IIb/IIIa inhibitors. In the PACT trial, for example, stents and abciximab were used in 26% and 5% of patients, respectively. Several other studies have investigated the role of GP IIb/IIIa antagonists in rescue PCI after failed fibrinolysis.78 The SPEED trial randomized 528 AMI patients to abciximab alone, reteplase alone, and various combination of reduce-dose reteplase with abciximab and PCI was performed in most patients. Early PCI was encouraged, and 323 patients (61%) underwent PCI at a median of 63 min after reperfusion therapy was started. Overall clinical success at 30 days (freedom from death, re-infarction, urgent revascularization, major bleeding, and transfusion) was achieved in 85% of patients undergoing early PCI. There were trends toward a lower composite end point of death, re-infarction and urgent revascularization in the combination group (reduce-dose reteplase with abciximab), without an increase in intracerebral hemorrhage. Patients undergoing early PCI had fewer ischemic events and bleeding complications (15% vs 30%, p=0.001).79 These results contrast with previous studies of angioplasty performed shortly after full-dose thrombolysis.80-82 Angioplasty techniques have advanced substantially since these trials, however, and they include the current widespread use of stents and potent antiplatelet therapy. In this regard, several trials have shown that abciximab can improve the results of primary angioplasty in AMI.83-86 In the RAPPORT trial, 483 patients with AMI were randomized to abciximab or placebo before PCI.87 The rates of death, or MI, and death, MI, or urgent revascularization were reduced from 11.2% to 8.7% and from 17.8% to 11.8% respectively. A similar 47% reduction in a combined end point was observed in the ADMIRAL trial, which also allowed stent implantation.88 The GUSTO III trial included a prospectively designed subgroup analysis to examine the effects of abciximab in 392 patients with AMI who underwent rescue PCI within 24 hs of fibrinolytic infusion. The adjusted 30-day mortality rate was lower in patients who received abciximab compare with those who did not (3.6% vs 9.7%, p=0.042). However, there were also a trend toward higher rates of major bleeding in the abciximab group (3.6% vs 1.0%, p=0.08).89 The specific fibrinolytic agent used may play a role in the improved outcome achieved. Reteplase has been shown to produce greater early TIMI grade 3 flow compared with alteplase.90 Although the GUSTO III trial showed no difference in mortality rates of patients with MI treated with reteplase versus alteplase,91 patients undergoing rescue PCI wih abciximab had improved outcomes when they had been randomized to reteplase compared to alteplase.89 The STOPAMI 2 trial randomized 162 MI patients to stenting plus abciximab and to alteplase plus abciximab to compared clinical outcomes and myocardial salvage.92 The combined incidence of death and recurrent MI was 7.4% in the stent and 17.3% in the alteplase group, relative risk 0.40(95% CI 0.16-1.01), p=0.053. Salvage index was significantly greater in the stent group (median 0.60, 25th, 75th percentiles: 0.37, 0.82) than in the alteplase group (median 0.41, 0.13, 0.58), p=0.001. In patients with AMI, stenting plus abciximab increases the chances of a favorable clinical outcome as compared to alteplase plus abciximab. The significant degree of myocardial salvage is the mechanism of the clinical benefit yielding by stenting plus abciximab.92 Abciximab was also shown to significantly improve myocardial microcirculation reperfusion and salvage, assessed by myocardial blush grade, after PCI for acute ST-elevation MI.93
The INTRO-AMI trial with 649 patients demonstrated that eptifibatide-mediated inhibition of platelet aggregation in conjunction with half-dose t-PA enhances the TIMI flow grade 3, and even more importantly, speeds the process of reperfusion, such that most of the effect is already observed by 60 min.94 Eptifibatide was administered in a double-bolus manner (10min apart). The single-bolus regimen of 180μg/kg achieves the minimum 1,600 ng/ml plasma concentration which results in the desired level of receptor occupancy and inhibition of platelet aggregation of at least 80% of baseline. However, it is frequently accompanied by a transient decrease in drug concentration (below 1,600 ng/ml at 30 to 60 min) following the initial bolus.95,96 There were 346 patients whom underwent PCI in the INTRO-AMI trial. The TIMI flow grade 3 observed compares favorably with other combination regimes and with data from larger studies of primary angioplasty.
What about the concomitant use of two platelets GP IIb/IIIa receptor antagonists in ACS? Following the PRISM PLUS and PURSUIT trials, there has been an increasing usage of tirofiban and eptifibatide to treat ACS patients in the early phase of their clinical course. Some of these patients later require PCI and may benefit from the long-term improvement in outcomes that had been documented with abciximab. In this regard, Lev et al demonstrated that administration of abciximab immediately after tirofiban or eptifibatide therapy effectively inhibits platelet function in a safety manner.97 Therefore, the conformational changes that the small molecule drugs induce in the platelet GP IIb/IIIa complex does not interfere with subsequent binding of abciximab to the GP IIb/IIIa receptor. Thus, the presence of a small molecule GP IIb/IIIa inhibitor would not impede the inhibitory effect of abciximab on platelet function in patients undergoing subsequent PCI. Future randomized, controlled trials would shed further light on this manner.
A planned strategy of all 3 treatments produces patency rates seen with primary and rescue angioplasty without sacrificing the critical early time benefits of pharmacologic therapy. Facilitated PCI may further improve outcomes by speeding the time to vessel patency. In this endeavor, the FINESSE trial randomized patients with AMI to facilitated PCI (reduced-dose reteplase, abciximab, and planned immediate PCI) or primary PCI with the use of abciximab at the time of intervention. They tested the hypothesis that facilitated PCI as a strategy for the management of myocardial infarction will be superior to primary PCI in higher risk patients. However, Ellis SG et al.,98 observed that the FINESSE Trial failed to prove this hypothesis. They concluded that neither, facilitation of PCI with reteplase plus abciximab nor facilitation with abciximab alone significantly improved the clinical outcomes, as compared with abciximab given at the time of PCI, in patients with ST-segment elevation myocardial infarction (Figure 4).98

Figure 4 Kaplan−Meier estimates of the proportion of patients with the composite end point.
The composite end point in the FINESSE trial included death from all causes and complications of myocardial infarction from randomization through day 90. Data are shown for all patients randomly assigned to a treatment group. P=0.55 for the comparison of primary percutaneous coronary intervention (PCI) with reteplase-plus-abciximab–facilitated (combination-facilitated) PCI. P=0.86 for the comparison of primary PCI with abciximab-facilitated PCI. P=0.68 for the comparison of abciximab-facilitated PCI with combination-facilitated PCI.
Reprinted with permission from Ellis SG, Tendera M, de Belder MA, et al. For the FINESSE investigators. A clinical trial of facilitated PCI in ST elevation myocardial infarction. N Engl J Med. 2008;358:2205-2217.
As Gibson CM stated, there is a growing body of AMI literature supporting a “union in reperfusion”: the use of pharmacologic agents to quickly open both the artery and the microvasculature and the use of adjunctive interventions to further improve flow and keep arteries open.99 Furthermore, with the addition of using an emboli protection filter device plus a multifaceted pharmacological approach,100-105 plaque debris could be retrieved reducing the incidence of embolization to the distal circulation.106-110 These measures are particularly interesting in saphenous vein grafts PCI, which are known to be associated with worse outcome compared to intervention of native coronary arteries,111-113 a setting where abciximab has shown no benefit.114,115 It is always nice and rewarding to see that there is so much work done on this field, but even nicer to know that there is still a long way to go with further investigations to find some light on yet unanswered questions.
Intravenous platelet GP IIb/IIIa receptor antagonists represent a significant advance in the practice of interventional cardiology and have proven to be an important, clinically effective adjunct therapy during PCI. The additional beneficial effects are most apparent in those patients with ACS with evidence of myocardial damage or who are undergoing early PCI.116-118 These agents have been a useful addition to the therapeutic armamentarium in this setting.119-123 The benefits of this therapy should certainly be provided to patients at elevated risk for procedural complications.120 Adding Platelet GP IIb/IIIa receptor inhibition therapy to coronary stenting implantation has demonstrated to have short and long-term reduction in ischemic complications, improved microcirculatory function and recovery of myocardial function, and better prevention of the development of restenosis in diabetic patients and reduced long-term mortality.
Suboptimal levels of GP IIb/IIIa receptor antagonists are not only non-protective and pro-thrombotic, but they also promote inflammation. However, in the right clinical indications with adequate dosage, intravenous GP IIb/IIIa inhibitors will continue to play an important role in the management of ACS, especially in the setting of PCI. Despite the heterogeneous outcome, these agents still have potential advantages in patients with high clinical risk but low bleeding risk. Adjunctive platelet GP IIb/IIIa receptor inhibition has proved beneficial effects in the setting of PCI, and still has a place as bailout therapy for periprocedural PCI complications in ACS patients, such as those patients with large thrombus, with stent thrombosis, and refractory no-reflow phenomenon following PCI.
None.
Author declares that there is no conflict of interest.

©2016 Centurión. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.