Journal of
eISSN: 2373-4396


Case Report Volume 4 Issue 3
1Cardiology Department, General Hospital of Nikea, Piraeus, Greece
2Cardiology Department, Great Western Hospital, Swindon, United Kingdom
Correspondence: Christos A Zivlas, Cardiology Department, Great Western Hospital, Wiltshire, SN3 6BB, Swindon, United Kingdom, Tel 0044 7774199134
Received: November 14, 2015 | Published: December 21, 2015
Citation: Zivlas CA. A lethal complication 19 years after bentall reoperation: was there any other alternative option using cutting-edge technology? J Cardiol Curr Res. 2015;4(3):119-122. DOI: 10.15406/jccr.2015.04.00144
Newer techniques of endovascular repair for complicated aortic arch aneurysms are evolving and should be an alternative option for high risk patients, in the acute setting. We report a case of an 81-year-old man, who was admitted due to dyspnoea, back pain and low level of consciousness. His medical history included a Bentall operation for aortic aneurysm and severe aortic regurgitation, conducted 29 years ago. Because of endocarditis, a second Bentall redo procedure was carried out, 10 years after. A CT-angiography revealed a massive perigraft aneurysm formation extended to the arch, compressing superior vena cava, right coronary artery, brachiocephalic artery, and graft detachment from distal anastomosis, but within the aneurysmal sac. Patient was characterized as high risk and rejected for surgery. He died 36 hours later. An extensive review of alternative treatment options is presented, applying the latest methods of endovascular stent-grafts.
Keywords: aortic arch, bentall operation, aneurysm, endovascular repair, stent-graft, rupture
ECG, electrocardiogram; RBBB, right bundle branch block; LAH, left anterior hemiblock; TTE, transthoracic echocardiography; SVC, superior vena cava; SBP, systolic blood pressure; RCA, right coronary artery
Over 4 decades have passed since Bentall and DeBono1 published their pioneer operation for complete replacement of the aortic valve and ascending aorta.1 Thus, the modification of this procedure has become routine nowadays, saving hundreds of patients.2 Rates of early mortality of the procedure have been improved due to acquired experience, and are estimated between 0.7% and 11.2% among different series.2,3 Emergency and older age are significant predictors for increased rates of mortality.4 Despite improvements regarding ascending and descending aorta pathologies, treatment of aneurysms or dissections which include the aortic arch remains a challenge, especially in the acute setting and for high risk patients. Advances in stent-graft technology are expected to fill this niche next years. Endovascular treatment of acute aortic syndromes involving the aortic arch may reduce morbidity and mortality and exhibit fewer complications than open surgery.5
An 81-year-oldman presented to the emergency department due to dyspnoea, back pain and cognitive impairment. Twenty nine years ago, he had undergone aortic root and valve replacement with the Bentall-de Bono continuous-suture wrap-inclusion technique because of an aneurysm of the ascending aorta and severe aortic valve regurgitation. Ten years after the first operation, a redo Bentall operation was carried out, because of prosthetic valve endocarditis. A new aortic composite prosthesis was then used and the aortic wall was re-wrapped around the new graft. Patient’s medical history also included diabetes type II.
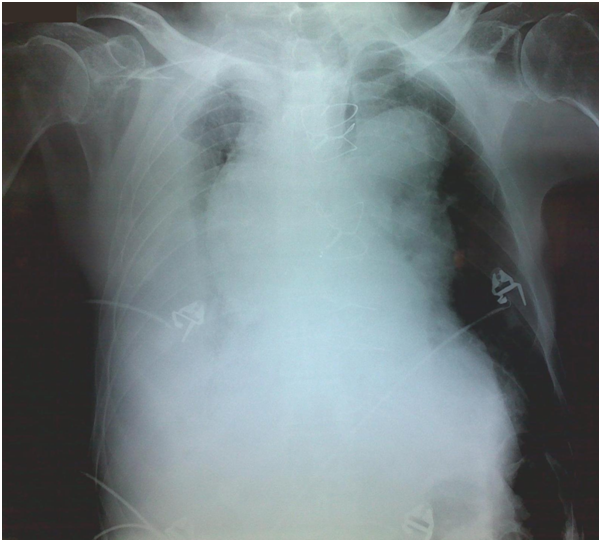
Clinical examination on admission revealed low blood pressure (80/50 mmHg), tachycardia (130 beats per minute) and hypoxia (oxygen saturation 90% with nasal cannula). Auscultation of the lung fields exhibited wet rales and absence of respiratory sounds, mainly at the base of the right lung. Heart sounds were muffled, however the closing click of the prosthetic aortic valve was distinct. The electrocardiogram (ECG) showed multiple disturbances, i.e. sinus tachycardia, right bundle branch block (RBBB) pattern, left anterior hemiblock (LAH) pattern, and T-wave inversion at infero-lateral leads. Jugular venous distention was also noticeable. A chest X-ray revealed an extensive mediastinum widening, aortic knuckle dilatation and right pleural effusion (Figure 1). A rapid transthoracic echocardiography (TTE) carried out in the emergency room confirmed that the proximal edge of the prosthesis was firmly attached to the left ventricular outflow tract, and the aortic valve was properly working. Blood tests demonstrated anemia (Hb 8.2 gr/dl), mild renal impairment (creatinine clearance 84 ml/min) and a slight rise of troponin I levels (0.55 ng/ml).
Due to patient’s history, a CT-angiography was immediately performed, which showed a massive perigraft aneurysm, 9x9.5 cm in maximum diameter, extended to the arch, compressing superior vena cava (SVC), brachiocephalic artery, right coronary artery (RCA), and graft partially detached from distal anastomosis, but within the aneurysmal sac Figure (2-7). The aneurysm was probably formed by a leakage at distal anastomosis, between the graft and the native aortic wall which was wrapped around it. A large right pleural effusion was also obvious. However, active leakage of the contrast agent from aneurysmal sac to the surrounding tissues was not observed at that point.
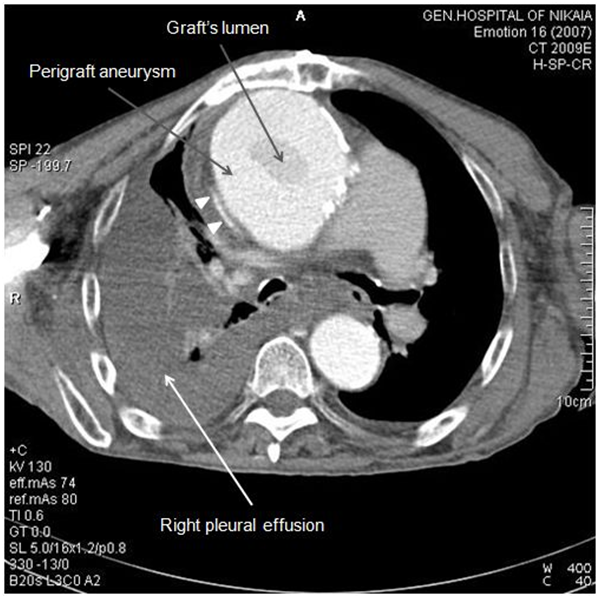
Figure 2 CT-angiography revealed a massive perigraft aneurysm, 9x9.5 cm. Superior vena cava compression (white arrowheads) and right pleural effusion are also manifested.
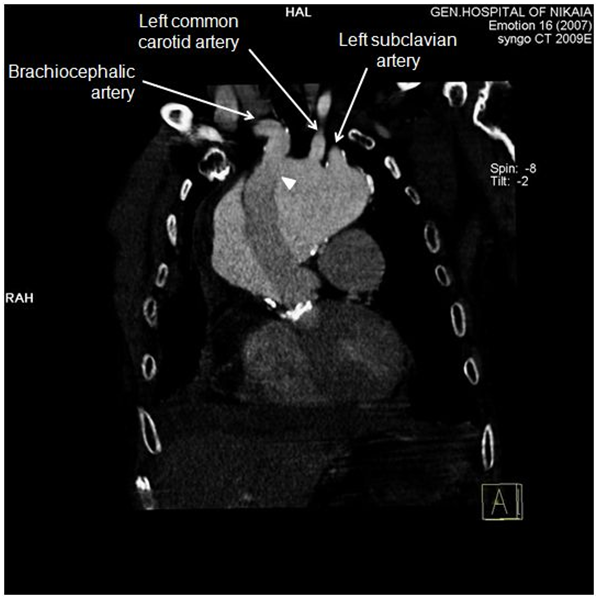
Figure 4 CT-angiography, anterior frontal view: Graft was partially detached from distal anastomosis (white arrowhead), resulting in perigraft leakage. Epiaortic vessels are visualized in this view. Proximal edge of the graft seems to be firmly attached to left ventricular outflow tract.
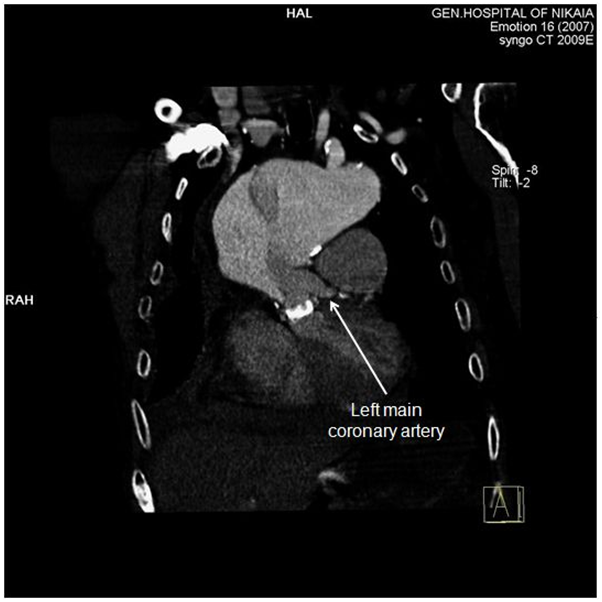
Figure 5 CT-angiography, anterior frontal view: Left main coronary artery is clearly demonstrated, while right coronary artery is compromised by the aneurysm.
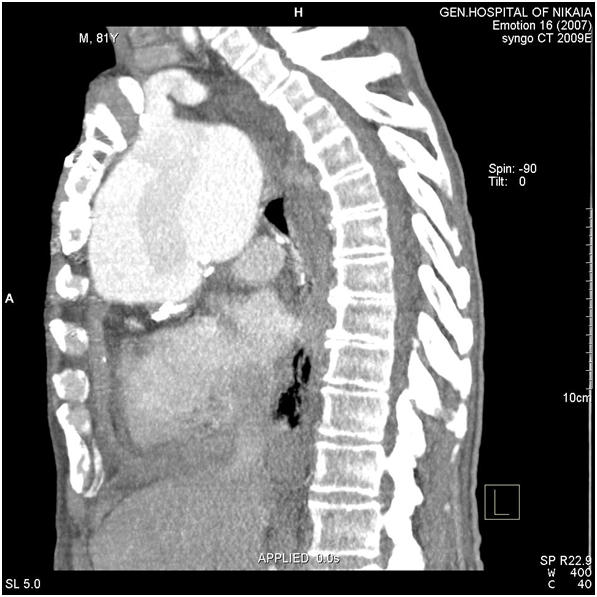
Figure 6 CT-angiography, lateral view: Aneurysm’s size and its adjacency to brachiocephalic artery and surrounding structures are depicted.
The patient was rejected for surgery and was immediately transferred to the coronary care unit. Initiation of a β-blocker agent, intravenous administration of normal saline and transfusion of one unit of packed red blood cells, partially improved patient’s hemodynamic condition. However, 36 hours later he developed ECG signs of acute myocardial infarction of the inferior wall and died. During these hours we re-evaluated any alternative surgical or endovascular therapeutic options. Patient’s pathology was considered as very high risk for surgery by our multidisciplinary team and experimental endovascular approaches for treatment of aortic arch aneurysms were not applicable to any of our referral cardiac surgery centers. Right pleural drainage, just before patient died, revealed hemorrhagic fluid.
We describe a rare case of an elderly patient with a lethal complication of a perigraft aneurysm formation, 19 years after a Bentall redo operation. Death was probably due to rupture of the aneurysmal sac, as advocated by hemorrhagic drainage of the right pleura, as well as heart and brain malperfusion. The exact time when the aneurysm was formed is obscure. A CT-scan of the chest, 4 years ago, did not imply any aneurysm recurrence, nevertheless, patient did not follow the recommendations of a yearly TTE, as suggested by most recent guidelines on aortic pathologies after repair or treatment.6 However, we suppose that this was a recent event, because of acute onset of symptoms.
The beginning of enlargement of residual aorta after repair of acute type A dissections has been shown to be unpredictable and may occur even after 10 years or more, postoperatively. Serial follow-up in these patients is therefore of utmost importance. Independent predictors for segmental growth of residual aorta include advanced age, aortic diameter, elevated systolic blood pressure (SBP) at late follow-up and patent false lumen. Marfan syndrome, nonresected primary tear, absence of postoperative β-blocker therapy and elevated SBP at late follow-up are independent predictors of late reoperation in these patients.7 Apart from advanced age, our patient did not have any other risk factor of the ones mentioned above.
No matter when perigraft aneurysm formation occurred, inclusion-wrap technique prevented an acute rupture to mediastinum and subsequent massive internal hemorrhage from distal anastomosis, for 36 hours. This provided invaluable time for searching alternative treatment options for such a high risk patient. Kouchoukoset et al.8 have reported a frequency of pseudoaneurysm formation after the inclusion-wrap technique from 7% to 25%, whose repair is associated with substantial risk. They suggest that the modifications of the original technique of Bentall and DeBono1 should minimize the need for wrapping the aorta around the graft. Acquired surgical experience with imbricated stitches and use of fibrin glue seem to lower hemorrhagic complication of the Bentall procedure recent years.9
Delay for intervention (surgical or endovascular) to this patient was proved fatal. However, surgical delay has been proposed over a decade ago for patients with type A dissection with malperfusion, dead or near-dead heart, limb, or visceral organ.10 Yet, there is a lot of controversy over this issue, and no solid guidelines exist, regarding malperfusion syndrome and acute type A aortic dissection.6 Advances in endovascular therapeutics will definitely change this field in the near future. Our patient actually had myocardial and brain malperfusion, demonstrated by electrocardiographic changes, rise of cardiac troponin I levels and cognitive impairment. Malperfusion of the heart derived from dynamic obstruction of RCA by the massive pseudoaneurysm (Figure 5), while malperfusion of the brain was caused by dynamic compression of brachiocephalic artery. Moreover, SVC syndrome was induced by pseudoaneurysm compression. As no active extravasation of the contrast agent from the aneurysmal sac to the surrounding tissues was visible at the time of CT-angiography, it is reasonable to imply that right pleural effusion at that time was transudate and produced by high central venous pressure at upper body.
What were treatment options for this patient? Was it possible to avoid this lethal complication using every available technology in the field? And furthermore, was it possible to predict when aneurysm rupture would occur?
Obviously, the first treatment option that was considered for our patient was immediate open surgery, with reconstruction of the entire arch aorta with an aortic graft with multiple prefabricated side branches, under deep hypothermic circulatory arrest, with selective antegrade cerebral perfusion. The pseudoaneurysm would be resected, releasing obstructed vessels, e.g. RCA, brachiocephalic artery and SVC. The old valved graft would remain in situ and an end-to-end anastomosis with the new branched graft would be conducted. Considering hemodynamic state and malperfusion of our patient, perioperative mortality would be very high. Deed et al. have shown a mortality rate up to 89% in such patients.10
Minimally invasive and hybrid endovascular techniques seemed to be more favorable for our patient. The “frozen elephant trunk” procedure would be feasible in this case, minimizing time and complexity of operation. Favorable results have been described with this technique recently.11 However, cardiopulmonary bypass, aortic arch opening and selective antegrade cerebral perfusion would be required again.
All the previously mentioned would be avoided with a hybrid technique for total arch repair with transposition of the supra-aortic vessels to the ascending portion of the old graft and a stent-graft deployment through a femoral artery. With this technique we would take advantage of the safe, non expandable proximal landing zone of the old graft, where the new endograft would be attached. Antona et al.12 have demonstrated the feasibility of this technique in a small number of high-risk patients with aortic arch aneurysms. They reshaped the proximal landing zone of the aorta with a vascular prosthesis, for safer fixation of the endograft, after epiaortic arch debranching. Cardiocirculatory arrest wasn’t carried out, because aorta was tangentially clamped for end-to-side anastomosis of the prosthesis for supra-aortic vessels. Nevertheless, patient’s chest is surgically opened again with this technique.
We believe that a total endovascular reconstruction of the aorta and arch was the treatment of choice for this patient. Although in yet early stages of development and experimental feature of the method, new methods of stent-grafts offer this treatment option for high risk patients. Inoue single- and triple-branched stent-grafts have been implanted in older aged patients (>70 years) with coexisting morbid conditions.13 Authors have reported satisfactory results of complete exclusion of thoracic aneurysms in 60% of their patients’ group. This technology, in contrast to surgery, promises shortened hospital stays, avoids the need for open surgery and its complications, and potentially lowers costs. However, Inoue stent-grafts are custom-made and individually designed in order to fit to each patient’s arch anatomy. Therefore, they are not suitable for acute situations, as our patient. This technology is expected to overcome its disadvantages, with computer-aided development of branched stent-grafts with dimensional adaptability, in order to be used for acute dissections or aneurysm ruptures of the aortic arch.
Currently available in situ stent-graft fenestration technique recently described by Sonesson et al.14 seemed to be the best treatment option for our patient. The only difference in our patient would be proximal deployment of the stent-graft into the old graft of the ascending aorta (Figure 8). With this innovative technique patient’s chest would be neither surgically opened, nor a cardiopulmonary bypass would be conducted. Only a temporary bypass from a femoral artery to the carotids with cooled blood would be necessary, so as cerebrum to be protected. Because the arch was aneurysmatic, covered stents would seem to be more attractive for our case, in order to seal epiaortic arch vessels. Last but not least, regarding the compromise of RCA, it would be possible to place a bare metal stent in the ostium of the vessel. With this hypothetic but to our opinion feasible approach, patient would be offered a simple and complete endovascular solution and a second hope.
Finally, novel technology gives answer to the question whether it was possible to predict when aneurysm rupture would occur. Remote wireless pressure sensing for endovascular leak detection after endovascular thoracic aneurysm repair has been described recently.15 Since our patient was not followed up so closely as guidelines suggest,6 placing an aneurysm sac pulse pressure device during surgery would be a prudent action. However, this technology was not available even at the time when the second Bentall operation was conducted, 19 years ago. Furthermore, echocardiography cannot visualize distinctly aortic arch pathology, while serial CT-scans render high dose of radiation and renal dye exposure. We believe that in difficult cases of high risk patients with aortic arch aneurysms, placing a remote wireless pressure sensing device is therefore of utmost importance. It also abrogates the need for costly and time-consuming magnetic resonance scans. Improvements in this field of patients’ monitoring after endovascular thoracic aneurysm repair are eagerly expected.
Very late complications following a modified Bentall operation should be expected, predisposing to very high mortality rates, especially if malperfusion syndrome occurs.10 Advances in hemostatic modifications are anticipated to improve outcomes.9 Serial follow-up with appropriate imaging methods should never be neglected. Novel endovascular techniques of stent-grafts offer a wide range of alternative options for the treatment of aneurysms and dissections involving ascending aorta and arch in high risk patients. Even though these techniques are not approved in latest guidelines for routine use,6 they should be considered in highly selected patients, for which open surgery is expected to have significant mortality risk. In situ stent-graft fenestration technique for arch vessels has been applied14 and seems to be a simple and feasible solution, when other conventional methods of treatment have been rejected. Randomized prospective clinical trials comparing various endovascular techniques for the treatment of ascending aorta and arch pathologies are highly needed.

©2015 Zivlas. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.