Journal of
eISSN: 2373-4396


Research Article Volume 4 Issue 3
1ACURE Biotechnology, INC., USA
2Department of Neurology, The Second Hospital of Hebei University, China
Correspondence: Xin Wang, ACURE Biotechnology, INC., Ellicott City, Maryland, USA
Received: December 15, 2015 | Published: December 16, 2015
Citation: Wang X, Zhang Y, Meng R, et al. Dexamethasone restores the neovascularization of spine cord in experimental autoimmune encephalomyelitis (EAE) rodent models. J Cardiol Curr Res. 2015;4(3):119-122. DOI: 10.15406/jccr.2015.04.00142
Nerves and blood vessels are completely different original tissue types and have distinct patterns in development and discrete features in anatomy and cellular biology. Stroke is a group of vascular diseases occurred in brain. Currently no reports show effective treatments restoring the blood vessels after injury in centre nerve system (CNS). Backed by my 20 years medical research experiences, I hypothesis that the lateral circulation could be induced through neovascularization by employing hormone at the sub acute stage of stroke onset while the inflammations initiating in brain tissue. This study employs Experimental Autoimmune Encephalomyelitis (EAE) models to show evidence that dexamethasone, a corticosteroid, restore the neurovascular units, also called blood-brain barrier (BBB), in spine cord through neovascularization. We discuss that pericytes are the key for neovascularization. In conclusion, application of hormone induces neovascularization in damaged centre nerve system tissue, has potential to establish effective lateral circulation after brain injury, including but not limited in stroke.
Keywords: neurovascular units (also called blood-brain barrier, bbb), pericytes, neovascularization, inflammation, hormone therapy, centre nerve system (CNS), stroke
Nerves and blood vessels are completely different original tissue types and have distinct patterns in development and discrete features in anatomy and cellular biology. Stroke is a group of vascular diseases occurred in brain. Although stroke appears neurologic function damage in centre neuron system, actually vascular deficits in brain, heart and other parts of body, even sometimes in kidney are the origin etiologies of stroke. May be this is the reason that neuron protective treatments have not found significant effects on outcome of stroke. AHA guideline brought out slogan “time is brain” on current therapeutic strategy in order to reduce morbidity and disability consequence of Stroke. Stroke Conference 2015 adds the effective endovascular intervention options into guidelines for the early management of selected patients with acute ischemic stroke, nonetheless, the dangerous side-effects of interventional treatments cannot be ignored. Backed on my 20 years medical research experiences, I hypothesis that the lateral circulation could be induced through neovascularization with physics, like hypoxic microenvironment, combine with chemicals such as hormone employing at the subacute stage of stroke onset while the inflammation initiating in brain tissue. This study employs Experimental Autoimmune Encephalomyelitis (EAE) models to show the evidence and discuss the potential mechanism to support this theory.
C57BL/6 female adult mice (n = 20) were used in this study. The animals were randomly divided into five groups four mice each for two time points of EAE, DXM group and one control group. The handling of animal is approved by Office of Scientific Research Ethics Management of the Second Hospital, Hebei Medical University.
Routine procedure for experimental autoimmune encephalomyelitis (EAE) was used. Briefly, mice are immunizated with 250μg of MOGp35-55 (lysine Bio-system, XiAn, China). All peptides were dissolved in complete Freund’s adjuvant (Sigma, St Louis, MO, USA) containing 4 mg/ml of heat-killed mycobacterium tuberculosis H37Ra (Difco Laboratories, Detroit, MI, USA). At day 0 and 48 h post immunization, C57BL/6 mice were injected with 500 ng of pertussis toxin (Alexis, San Diego, CA, USA) in PBS, intraperitoneally (i.p.). Mice were examined daily for clinical signs of EAE and scored as follows:
We decided the start day (E0) for treatment at the next day that paralysis scores become 2. Most of mice reached the peak of severest symptoms at 7th day after the start day (E7), the symptoms started getting mild after. We chose the 14th day after the start day as the end point of this study (E14). We studied the impact of Dexamethasone on the pathological changes of spine card in the process of outbreak of acute inflammation of spine cord through H&E staining.
The spine cords at E7 show typical asymmetric pathological characteristics of acute inflammation (Figure 1 10x): swollen of white matter of tissue; invasion of inflammation cells along with appearance of vascular sleeve; micro-vessels in spine cord significant shrivel and stripping from surrounding tissue; number of micro-vessels decreased, and even disappeared at E14 (A and left side of C in Figure 1). The B and D in Figure 1 are the treatment of dexamethasone at E7 and E14, respectively. The micro-vessels in spine cord tissue are siginificantly remained, and neovasculars clearly appeared (arrows). Under the low power microscope, the mice after dexamethasone treatment show significant maintaining of micro-vessels and newly formed neurovascular comparing to un-treated E7 mice, especially around the area of central tube of spine cord. The spine cords of E14 mice and rats that treated by dexamethasone show the enriched micro-vessels, whereas no micro-vessels could be found in untreated E14 mice. These results suggest that dexamethasone restores the neovascularization in inflammatory centre neuron system tissue spine cords.
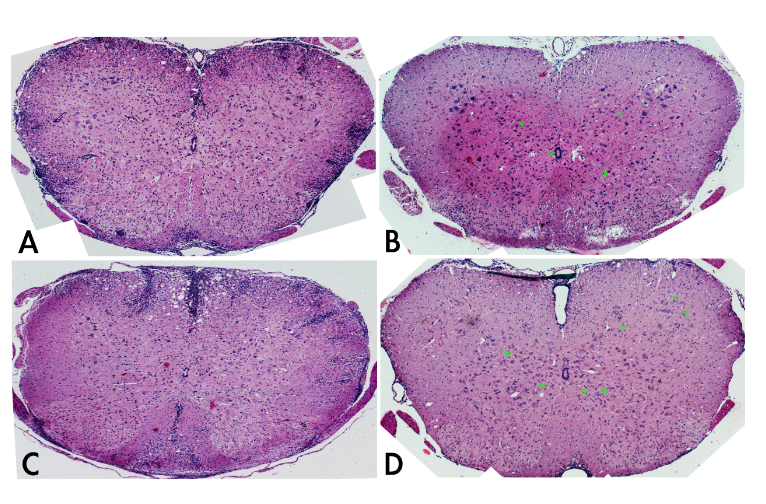
Figure 1 Cross section of spine cords of EAE mice with/without Dexamethasone treatment under the low power microscope (10x).
Under the high power microcopy (Figure 2 40x), dexamethasone treated mice clearly filled with neurovascular units, single endovasculium and pericytes (Stars) formed neovascularization in spine cord at both E7(B) and E14(D), whereas no any neurovascular units can be seen at all in spine cord of EAE mice models. At E14, newly formed neurovascular units are more mature comparing to at E7.
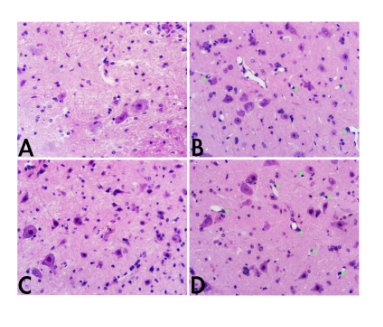
Figure 2 Spine cords of EAE mice with/without Dexamethasone treatment under the high power microscope (40x).
Neurovascular unit is also called blood-brain barrier (BBB). Elements of neurovascular units include endothelium, pericytes and astrocytes, neurons. Del Zoppo GJ1 brought the “neurovascular unit” concept on the table for stroke, but the targeting clinical trials addressed only to neuron protection.1 Our study here is the first time to focus on restoring neovascularization of neurovascular units, and successfully provided clear feasible evidence for rebuilding neovascularization in center nerve system. The application of this treatment will be expected to restore the neurological function of brain after stroke.
Dexamethasone has been used in the therapy of acute multiple sclerosis (MS), multiple myeloma, drug-eluting vascular stents as a drug of anti-inflammation and immune suppression. However, the main mechanism of dexamethasone is unclear. In our previous study, modification of hormone affected the function of pericytes in prostate glands.2 Under chronic inflammation, cytokines such as VEGF, IL17, induced transformation of cell types and neovascularization involving pericytes in periodontitis rats.3 Hormone treatments showed increased expression of HIFs in human metastatic cancer cell lines (data not published), while hypoxia increased mobility of actin positive cells as well as pericytes.4 Hormone therapy is dangerous for patients with brain metastatic Triple Negative Breast Cancer.5 Taken together, the mechanism of neovascularization in center nerve system induced by Dexamethasone in this study may be that hormone stabilized the microenvironment of injured area of CNS, protected or activated the transformed pericytes from inflammation cells. Pericytes localized and became the first occupant cells recruiting transformed endothelium for formation of neovascularization in gray and white matter of injured CNS.

©2015 Wang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.