Journal of
eISSN: 2373-4396


Case Report Volume 2 Issue 5
Department of Cardiovascular Magnetic Resonance, Department of Echocardiography Argentina
Correspondence: Ricardo Obregón, Echocardiography Department, Instituto de Cardiología de Corrientes, Argentina
Received: April 23, 2015 | Published: May 14, 2015
Citation: Obregón R. Non compaction of the ventricular myocardium: a logical reasoning. J Cardiol Curr Res. 2015;2(5):119-122. DOI: 10.15406/jccr.2015.02.00077
Non-compaction myocardium is a common finding in recent years. We explain the different diagnostic methods that we have for diagnosis, and we analyze their similarities and differences. Besides its clinical presentation, prognosis and clinical relevance are not clear yet. We present six different cases and we review the literature. We still don´t have all the answers, but we can´t leave without non-compaction myocardium diagnosis.
Keywords: left ventricular non compaction, genetic heterogeneity, cardiomyopathy, echocardiography, cardiac magnetic resonance imaging
DCM, dilated cardiomyopathy; CMR, cardiovascular magnetic resonance; HCM, hypertrophic cardiomyopathy
The concept of “non compaction of the ventricular myocardium” (LVNC) has gained increasing participation in scientific literature in the last years. From the beginning the “epidemic” propagation of this entity may be considered the same as mitral valve prolapse in the 80’s. Actually and, even though its profound analysis, LVNC was not defined as a pathological entity itself, which is the reason that cardiomyopathy guidelines considered LVNC as a "unclassified cardiomyopathy".1 For better understanding of anatomical findings, it is necessary to know normal myocardial structure and formation. During embryonic development of the left ventricle we find a completely spongy myocardium. Myocardial perfusion is supplied, according to this structure, directly by the blood of the ventricular cavity. During the 5th through the 8th weeks of gestation, a myocardial compaction process starts in the epicardium and progresses inward towards the endocardium, from the base of the heart to apex. In adults thin apical endocardial trabeculae can persist in the left ventricle, as memory of this process, and this finding is more evident on the walls of the right ventricle.
Non-compaction myocardium defines the "excessive ventricular trabeculation" in the subendocardium, and this process is persistent and very different from a thin compact layer of epicardial muscle. One of the more accepted theories says this result comes from an arrest of the normal process of myocardial compaction during embryonic development.2 When this arrest occurs, the ventricle wall has a predominant trabecular aspect, mainly distributed in the medium-apical left ventricular segments, creating an image similar to a sponge”, hence the term "spongy myocardium". These morphological changes also cause deep recesses from the ventricular cavity to the more superficial layers of the wall.3 Initially, these findings were considered normal variants, and they were simply referred as "ventricular hypertrabeculation" linking them with left ventricular hypertrophy. This conceptual association is partly true and partly not. LVNC has been observed in association with multiple cardiac pathologies in children4 and adults.5 The association observed with depressed ventricular systolic function, diastolic dysfunction, clinical events such as embolia6 and cardiac arrhythmias makes us think that this structural alteration is more related to pathology than normal.7–9
LVNC criteria
At least six different definitions have been proposed for LVNC diagnosis: four of them considered echocardiographic criteria,4,10–12 one definition is based on cardiovascular magnetic resonance (CMR) findings and the other in anatomopathology.13 The most frequently applied definition contains the Stӧllberger and Oechslin criteria used by Jenny et al.14 First, the presence of two different layers in the myocardium, with a relationship between the non-compacted layer and the compacted layer (NC/C) > 2.0 in adults and greater than 1.4 or 1.3 in infants.15 Wall segments are measured at end-systole on cardiac short axis. Second criterion: the presence of three or more prominent myocardial trabeculae located in relation to papillary muscles (apical) and the third criterion Color Doppler echocardiogram demonstrating flow within deep intertrabecular recesses in continuity with the left ventricular cavity.
The diagnostic criteria used by the CMR are similar to those used by echocardiography, but the measurements are made with ventricle at end-systole. On the other hand the CMR study not only the short axis, but also evaluate the ventricular long axis (two cameras, four cameras and the one that includes left ventricular outflow tract view). The relationship between NC/C layer is different according to the ventricular section where this alteration is observed. A NC/C ratio of more than 2.3 in diastole, observed in the ventricular short axis has a diagnostic sensitivity and specificity of 86% and 99% respectively.16 We have diagnosed LVNC using this last criterion in our cardiology center. Some patients are examples of clinical diversity that can be observed in daily practice.
Case 1
80 years old patient with no known cardiovascular risk factors had been symptomatic for dyspnea from long time ago and physical examination evidenced cardiomegaly. The electrocardiogram showed incomplete left bundle branch block. Echocardiography demonstrates trabeculations, ventricular enlargement and prominent myocardial walls recesses (Figure 1). In this case LVNC has had a good clinical outcome, due patient’s age. Bad news for adult patients with heart failure and LVNC is that when this association is diagnosed can only have two evolutions, to stabilize or get worse. When left ventricular dilation and functional impairment are present, the annual death rate is about 35%, same mortality as idiopathic dilated cardiomyopathy.17 Definitely, prognostic factors are not different from dilated cardiomyopathy. Ejection fraction deterioration and symptoms as major predictors of poor outcome. Comments: LVNC observed in advanced stages of life, associated to ventricular dilation and impaired systolic function has the same prognosis than idiopathic dilated cardiomyopathy (DCM).
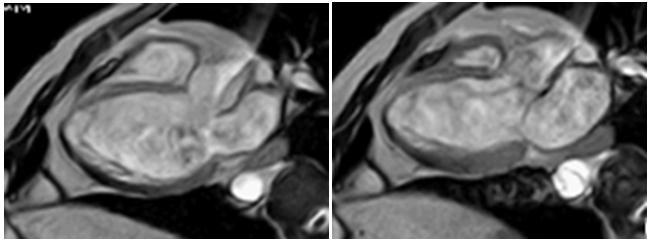
Figure 1 CMR with Fast Field Echo sequence. Left image: the heart in diastole, shows left ventricular hypertrabeculation. Right image: In systole (with systolic dysfunction) absence of contraction in the trabecular region is observed. The ventricular contractile function is maintained from the basal segments.
Case 2
31-years-old patient with a family history of sudden death admitted to medical ward due chest pain and electrocardiographic changes. A surface echocardiogram diagnosed asymmetric hypertrophic cardiomyopathy (HCM) with a basal septum of 17 mm and thinning of the basal lateral wall with LVNC criteria in that segment. The presence of hyper trabeculations in patients with left ventricular hypertrophy is one of the most frequent findings. When LVNC criteria were found “somewhere" on a ventricular segment this finding should make us think about three possible diagnosis: ventricular crypts, LVNC undulating phenotype or secondary left ventricular hypertrophy. All these entities have been associated with LVNC.
Ventricular crypts: These alterations are characterized by deep recesses usually located in the middle and basal segments of the inferior wall of the left ventricle as in the inferior interventricular septum where it contacts both ventricles (Figure 2). The crypts are displayed in the images (obtained by Eco, CMR and CT) at end-diastole because the ventricular contraction collapses them during systole (Figure 3,4). In the classic work of Germans et al.,18 16 patients with genetic susceptibility to HCM were compared to 16 healthy volunteers. The authors observed that ventricular crypts were in the 81% of patients with genetic susceptibility. This finding shows that ventricular crypts observation by CMR, is an effective way of early diagnosis in the HCM and can detect it even before the typical ventricular hypertrophy appears. The CMR has shown greater sensitivity than ultrasound to diagnose regional ventricular hypertrophy,19 and the same happens with the visualization of ventricular crypts. Anyway, when a CMR is performed, it is necessary to obtain multiple oblique views to diagnose the crypts in a precise way.
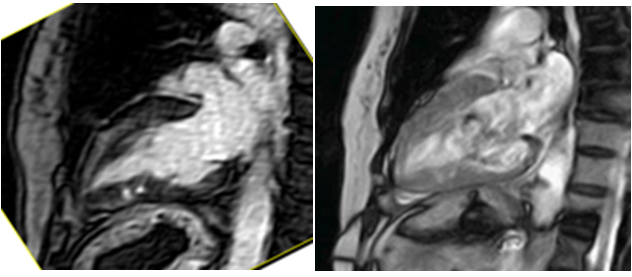
Figure 2 Left image: CMR T1 gadolinium-sagittal section (two cameras): heterogeneous uptake of contrast on the anterior wall with accumulation of contrast in the inferior wall associated with hyperlucency cine resonance images (gradient echo) on the right. These findings are compatible with ventricular crypts located on the inferior wall of the left ventricle, in the context of hypertrophic cardiomyopathy and hypertrabeculation.
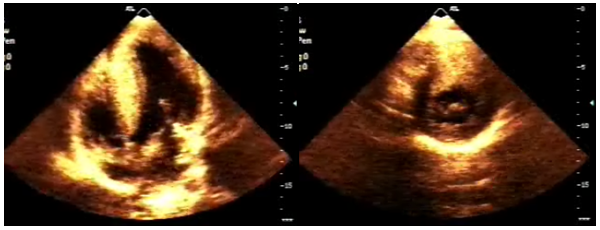
Figure 3 Echocardiogram in 2D mode. Left image in apical view with four cameras shows a significant hypertrophy of the entire septum. Lateral view in basal segment shows a significant myocardial thinning without contractility deterioration, possible "ventricular crypts". Right image, basal short axis is difficult to differentiate ventricular crypts seen in the previous Figure.
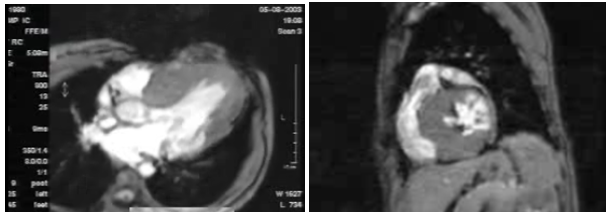
Figure 4 Same patient as in Figure 1. Left image, cardiac CMR. Sequence fast field echo (FFE) also called gradient sequence, TE / TR / flip: 7.7 / 2.9 / 15º, in an oblique cross-sections of the heart (ventricular long axis) predominant septal hypertrophy and trabecular thinning of posterior wall in basal segment called "ventricular crypts". Right image, with the same pulse sequence, basal short axis of the left ventricle ventricular crypts can be observed clearly; with compromise at lateral and posterior walls of the ventricle.
LVNC undulating phenotype: In this form LVNC is generally observed as a dilated ventricle with impaired ventricular function that evolves towards myocardial compaction and transient improvement in ejection fraction. This trend was first observed by Towbin and Bowles;20 Pignatelli evaluated 36 pediatric patients, 5 (13%) of them had an "undulating course" with a transient improvement in ejection fraction. This recovery was temporary, with further worsening ventricular function and presenting the same prognosis as the rest of the patients studied.
Left ventricular hypertrophy: The diagnosis of LVNC is difficult to make in patients with left ventricular hypertrophy due to another process. Patients with genetic susceptibility are likely to develop a LVNC when they are exposed to external factors that can cause secondary left ventricular hypertrophy (aortic stenosis, hypertension etc.) Gebhard et al.,21 published an interesting way to differentiate LVNC from secondary ventricular hypertrophy. This study compared 41 patients with moderate to severe aortic stenosis versus 41 patients with isolated LVNC and 41 normal subjects as a control group. The authors noted that hypertrabeculation was present in both groups studied but not in the control group. The best variable to distinguish LVNC from secondary ventricular hypertrophy was the maximum systolic thickening of the compact portion. When systolic thickening was < 8.1 mm this finding was specifically associated to LVNC, but if systolic thickening was greater than 8.1 mm the hypertrabeculation was associated with left ventricular hypertrophy secondary to aortic stenosis. In this line of research Jacquier et al..22 used CMR to compare the presence of LVNC in HCM and DCM. In this study they found that hypertrabeculation greater than 20% of total left ventricular mass is diagnostic of primary LVNC, with a sensitivity and specificity greater than 97% to differentiate from hypertrabeculation observed in HCM and DCM.
Comment: The evaluation of LVNC associated to left ventricular hypertrophy becomes relevant in patients at risk of sudden death, especially when the degree of parietal thickening does not meet all the criteria for HCM. In these patients, there should be a genetic study to confirm or rule out the causative gene for familial HCM.
Case 3
Dilated cardiomyopathy and LVNC: poor outcome?: 3-months-old patient with dilated cardiomyopathy and severe impairment of left ventricular function. Ventricular dilation observed on echocardiogram with prominent left ventricular hypertrabeculation, fulfilling the criteria of LVNC. The poor performance and the difficult clinical management prompted physicians to perform a CMR to evaluate myocarditis and/or LVNC. CMR was performed under general anesthesia and Gadolinium-DTPA contrast. Hypertrabeculation observed in the left ventricle involved the anterior and lateral wall in the middle and apical segments with severely impaired ejection fraction (Figure 5).
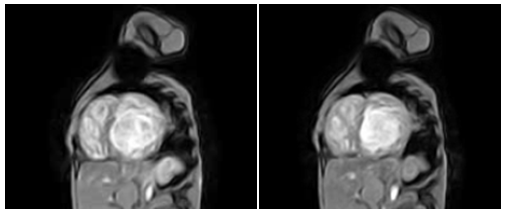
Figure 5 Image of fast field echo (gradient image) CMR short axis of both ventricles, left image in diastole and right image at systole. There is little change in volume in both ventricles due deteriorated ventricular function. Hypertrabeculation of both ventricles is evident.
Gadolinium-DTPA is used in CMR as a contrast to highlight areas of inflammation (early enhancement) and areas of fibrosis (delayed enhancement). The findings in LVNC are variable and they have not proved to be useful in this entity yet. The mismatch between the contrast enhancement and the evolution could be due to the lack of association between contrast uptake areas and hypertrabeculation. In three studies where gadolinium uptake was observed in LVNC, epicardial and right interventricular septum areas were observed distant from areas of hyper trabeculation.23- 25 These findings show us that fibrosis in LVNC is not the only cause of cardiac remodeling and impaired ventricular function. LVNC would, in these cases, only be an epiphenomenon within a larger process of myocardial fibrosis and inflammation. It has been observed also the presentation of LVNC in the evolution of dilated cardiomyopathy in an adult patient, as published by Hofer et al.,26 this could be a response to the significant subendocardial ischemia that occurs in dilated cardiomyopathy. When trying to achieve a compensating mechanism, the development of subendocardial trabeculae increase its surface, to take oxygen from the blood directly located in the ventricular cavity.
Comment: The young age of the patient suggests that rejecting "other" congenital disease and coronary abnormalities, the primary cause of the dilated cardiomyopathy should have a genetic susceptibility.27
Case 4
Myocarditis and LVNC: myocardial susceptibility: The association between LVNC and subendocardial fibrosis evidenced by CMR has led to the suggestion that an inflammatory process may be an “acquired” cause of these myocardial changes.28–30 However, not all patients diagnosed LVNC have myocarditis diagnosed by gadolinium uptake. So, myocarditis may suggest only an incidental association with LVNC, and this myocardium is possibly more likely than normal myocardium to inflammation in adverse conditions (Figure 6).
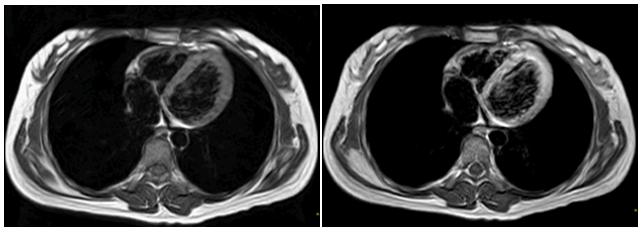
Figure 6 Left image: Heart Resonance T1-weighted transverse CMR image (without contrast) TE/TR: 40/200 ms, four heart chambers can be observed. The average myocardial signal intensity is 568. Right image: The same sequence but with gadolinium contrast to 0.2mmol / kg, the signal strength increase to 1234, with a difference of 2.17, demonstrating a significant increase in secondary myocardial signal, in this case, due myocarditis edema.
Comment: Gadolinium uptake in LVNC can be caused by two reasons, one is the enhancement in true subendocardial fibrosis in areas of hypertrabeculation, the other cause is blood stasis caused by the accumulation of contrast in the trabecular meshwork. Generally cardiovascular resonance takes an early gadolinium enhancement image (within 2 minutes) that shows regions with edema and / or blood stasis; while late gadolinium enhancement (images acquired between 10-20 minutes) defines the areas with myocardial fibrosis or myocardial necrosis.
Case 5
Child with dilated cardiomyopathy: 2 months-old patient with signs of heart failure. Echocardiography shows ventricular dilation associated to anterolateral LVNC and severe impairment of ejection fraction. CMR was requested and the diagnosis was confirmed.
The presence of LVNC in newborns has a genetic substrate. The studies show that only 50% of patients with LVNC have chromosomal abnormalities, but early age of onset of these morphological changes with ventricular dysfunction, give us few other etiological possibilities to justify this association. It has been identified G 4.5 gene (located in the locus of 11p and 15 chromosomes), as a cause of mutations in the coding of tafacina proteins (bound to the X chromosome), alpha dystrobrevin, ZASP, actin and lamins A / C. Of these mutations, the most frequently occurs in sarcomeric proteins: β-heavy chains of myosin. This genetic change cause, in some families, an autosomal dominant transmission. The presence of a "genotype" of LVNC does not define pathology itself. There have been found different "phenotypes" even in normal humans, with equal "genotypic" LVNC expression.31–33 It has been observed the development of LVNC in the evolution of patients with impaired left ventricular function, considering this LVNC as acquired. In patients with gene susceptibility they usually develop LVNC due to external noxa or an adaptation to subendocardial ischemia, hypertrabeculation the myocardium would be trying to take in more oxygen directly from the ventricular cavity.34 Infants with MNC may have different developments: the left ventricle may become dilated and dysfunctional, or compacted and improve its function, or have the “undulating” evolution and become a hypertrophic cardiomyopathy with uncertain prognosis.
Case 6
26 -year -old patient was referred for competitive evaluation. She underwent an echocardiogram showing LVNC criteria in the left ventricle. The bi-ventricular function, the strain and strain rate were normal. ECG and treadmill test and CMR were normal too. The presence of LVNC in normal patients varies depending on the "number of ventricular segments” evaluated to make the diagnosis; as it was proven in the MESA study which analyzed 1000 healthy subjects with resonance. When the criterion of relationship between the NC/C myocardium >2.3 was used in only "one-segment", the incidence of LVNC was 46% and declines to 6% when it was required the presence of hypertrabeculation in "more than one segment" (Figure 7,8).35
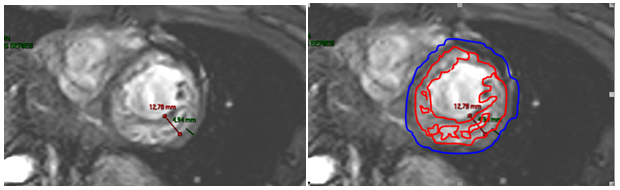
Figure 7 Left image: Cine CMR in short axis. Ventricular hypertrabeculation with a ratio between the compacted myocardial layer and noncompacted myocardial layer of 2.6, LVNC criterion. Right image: Same patient: ventricular mass compared to the mass of the compacted myocardial layer and non-compacted region (trabecular), in this case about 70%, LVNC diagnosis.
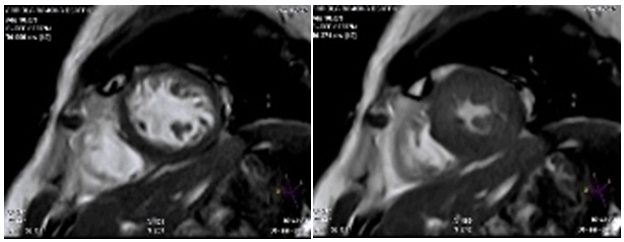
Figure 8 Left image: CMR with fast field echo sequence, short axis at papillary muscles. In diastole can be observed thickness of the compact portion of the ventricular wall about 11 mm, right the same image but at end systole, the wall thickness of the compact portion increases to 22 mm, achieving a thickness > 0.81 mm which is the criterion used to differentiate LVNC from ventricular hypertrophy.
Comment: More the over diagnosis of LVNC in a healthy patient can lead to a radical change in the quality of life and the patient stop playing sports. And also cause constant stress due serial evaluation of an illness that does not exist.
The best way to understand LVNC includes a preliminary evaluation about what population is under investigation. If you evaluate patients with dilated and deteriorated ventricular function (with high mortality risk) we probably come to the conclusion that LVNC is a predictor of bad outcome. Conversely, LVNC in a population of healthy people and athletes (with low mortality risk) will not have a bad prognosis.36 This is a diagnosis with variable and confusing data about its prognosis. What prognostic value has LNCV in "each" patient? This question can be answered only with the individual evaluation in each of the conditions in which LVNC is observed.
What can we say about LVNC? Can different pathologies cause changes in the evolution of LVNC? Hypertension, due to the increased after load may accentuates the production of myofibrils with subsequent hypertrophy; or genetic alterations (genotype) associated to LVNC sensitize the myocardium to be affected by infectious / inflammatory processes such as myocarditis. Are we in front of a different disease or just watching the behavior of a "thinner" myocardium exposed to afterload changes? Latest publications emphasizes systolic thickening in compacted myocardium more than in NC/C layer relationship.21
Author thanks Dr. Natalia Cocco to help in the translation of this manuscript.
The authors state that there is no conflict of interest.
None.

©2015 Obregón. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.