Journal of
eISSN: 2373-4396


Research Article Volume 2 Issue 5
Department of Cardiology, Argentina
Correspondence: Mariano Falconi, Department of Cardiology, Cardiovascular Imaging Section, Institute of Cardiovascular Medicine, Hospital Italiano de Buenos Aires, Peron 4190, Argentina, Tel 5491167578545
Received: April 13, 2015 | Published: April 27, 2015
Citation: Falconi M, Funes D, Arias A, et al. Dynamics of the left ventricular outflow tract during the cardiac cycle assessed (with) via tridimensional echocardiography. J Cardiol Curr Res. 2015;2(5):119-122. DOI: 10.15406/jccr.2015.02.00072
Objective: To analyze the left ventricular outflow tract (LVOT) dynamic during cardiac cycle with 3D Echocardiography (3DEcho).
Material and methods: We prospectively included 58 patients sent to our lab for a transesophageal echocardiographic study (TEE). All TEE were done with a 3DEcho probe, obtaining gated images (4 beats, complete volume) for off-line analysis. The cardiac cycle was divided in proto (S1), mid (S2) and telesystole (S3); and proto (D1), mid (D2) and telediastole (D3). The LVOT area was measured by planimetric method in short axis, orthogonal to the long axis of the LVOT in each phase.
Results: Mean age was 69.7±15 years, 60% male. LVOT area (cm2) in each phases was: S1: 4,36±1,02; S2: 4.08±0,94; S3: 3,82±0,93; D1: 3,59±0,98; D2: 3,77±1,01; D3: 4,15±1,11. Maximal area was observed in S1, corresponding to the closing position of the anterior leaflet of the mitral valve (ALM) and a few excursion of the interventricular septum (IVS) to the LVOT; minimum area was obtained in D1 coinciding with some persistence of IVS in the LVOT and maximal opening position of ALM. The total modification of the area was 17±12% (p<0.0001), changing also the shape of the LVOT from a partially elliptical or circular one (systole) to an markedly elliptical or semilunar (diastole).
Conclusion: the LVOT has a variation in area and shape during cardiac cycle, depending basically of the movement of IVS (systole) and mitral valve opening (diastole).
Keywords:Left ventricular out flow tract, Tridimensional echocardiography, Cardiac cycle
LVOT, Left Ventricular Outflow Tract; TEE, Transesophageal Echocardiographic; MSCT, Multislice Cardiac Tomography; 3DEcho, 3D Echocardiography; ALM, Anterior Leaflet of the Mitral valve
The present study prospectivelyincluded58 patients referred for transesophageal echocardiography, with 3D acquisitions of the left ventricular outflow to asses area and shape during the cardiac cycle. Area modification was observed by means of orthogonal cuts (maximun protosystolic and minimal protodiastolic for the area), and shape as well (partially elliptic or circular at systole and markedly elliptic or semilunar at diastole) mainly depending on the movement of interventricular septum and mitral anterior valve at the different phases of the cardiac cycle.
The left ventricle outflow tract (LVOT) is a structure that is limited mainly by the interventricular septum and the anterior mitral valve, towards where the systolic flow leads prior to its outflow by the aortic valve. Both limits are very mobile (particularly the anterior mitral valve) therefore it is expected that the LVOT area be modified both in its dimensions as well as in its shape during the cardiac cycle. It is relatively frequent that the LVOT be implicated in anatomical and anatomo-functional changes in pathological conditions (septal anterior systolic motion, basal septal hypertrophy, subaortic membranes). However, its normal dynamics are also important particularly in procedures that involve the area, such as percutaneous implant of aortic valvular prosthesis, where a variable portion of the protesic structure, depending on the model, rests on the LVOT. The objectives of the present research were to assess the modifications in the total area and LVOT shape during the cardiac cycle through transesophagic 3D echocardiography.
Between June 2010 and March 2011, 58 patients derived to perform a transesophageal echocardiographic procedure were included. All patients were older than 18 years and with sinus rythm at the moment of the study. Patients with structural or functional changes that could affect the LVOT dynamics such as history of percutaneous aortic valve implantation, heart surgery with pericardial opening, mitral or aortic valve replacement, alcohol septal ablation, anteroseptal infarct, presence of left bundle block, systolic anterior motion of the mitral valve, mitral stenosis, ventricular dysfunction (ejection fraction <50%), and frequent ventricular or supraventricular arrhythmia, were excluded. To perform the multiplanar transesophageal echocardiogram, a Philips IE 33 (Philips Ultrasound, Bothell WA, USA) was used, and an X7-2t transesophageal probe that allows to obtain multiplanar conventional images and different 3D acquisitions (in real time, real time zoom, complete volume, and orthogonal planes). LVOT analysis was carried out using “complete volume” images for post processing.
Studies were performed with topic anesthesia with spray lidocaine, after a minimun of 6 hours fasting, at left lateral decubitus posture. Patients were connected to the echocardiography equipment for continuous electrocardiographic monitoring to be able to obtain gated images. A conventional multiplanar echocardiographic study, and 3D images that were necessary to assess base line disease were obtained. At the end of the assessment, three series of complete volume 3D images in expiration apnea oriented to the LVOT acquisition center were obtained. In case any changes in the normal sinus heart rythm would appear (isolated extrasystolia in general), the series was deleted and a new one was obtained until three series without gating problems were obtained. Images paremeters were adjusted to achieve a frame rate of 15-20 fps.
To assess the LVOT area, a QLAB 7.1 software (Philips Ultrasound) was used at a work station that was separated from the equipment. Upon exportation of the complete volume series to the work station, LVOT orthogonal cuts were performed, paying particular attention to achieving an angle of 90° with its long axis. Consequently, the references of the planes provided by QLAB (usually red and blue planes) were used, thus obtaining a short axis of LVOT in the third plane (green) (Figure 1).
LVOT short axis was captured at 2-4 mm below the annular plane. Three systolic and three diastolic phases were considered: protosystole (S1): beginning of the aortic valve opening; mesosystole (S2): maximun opening of the aortic valve; telesystole (S3): beginning of the aortic valve closure; protodiastole (D1): beginning of the mitral valve opening; mesodiastole (D2): partial closure of the anterior mitral valve during diastasis; telediastole (D3): partial opening of the anterior mitral valve via the auricular systole. Due to the movement of the cardiac cycle, an unbalanced in the cut plane was caused; it was adjusted to achieve a short axis orthogonal to the LVOT long axis in each one of the 6 phases. After the corresponding short axis was obtained for each phase, the LVOT area was measured by planimetry, assessed in cm². An average of the three acquired cycles for each phase was obtained. The shape of the LVOT short axis was assessed by visual estimation and by the larger/shorter diameter relation during the protosystolic and protodiastolic phases. Values were expressed in media ± DS, median and ranges.
The mean age of the population was 69.7±15 years, and the reasons of the referrals were to evaluate valve disease (50%), embolic source (16%), arrhythmia (16%), suspected endocarditis (14%) or interauricular septal defect (3%). The rest of the characteristics are shown in Table 1. Planimetric measurement of the LVOT was feasible in 100% of the cases. A modification of the shape and area of the LVOT during the cardiac cycle was observed (Figure 2). The largest LVOT area was observed during protosystole, coincidentally with the scarce excursion of the septum towards the cavity and the closing position of the anterior mitral valve.
|
Characteristics |
Total = 58 |
|
Age (years) |
69.7±15 |
|
Males |
35 (60%) |
|
Hipertensive |
27 (47%) |
|
Dislipemic |
20 (34%) |
|
Diabetic |
5 (9%) |
|
Current smokers |
3 (5%) |
|
Moderate to sever aortic stenosis |
21 (36%) |
|
Moderate to severe aortic regurgitation |
8 (14%) |
|
Moderate to severe mitral regurgitation |
12 (21%) |
Table 1 Baseline characteristics of the population
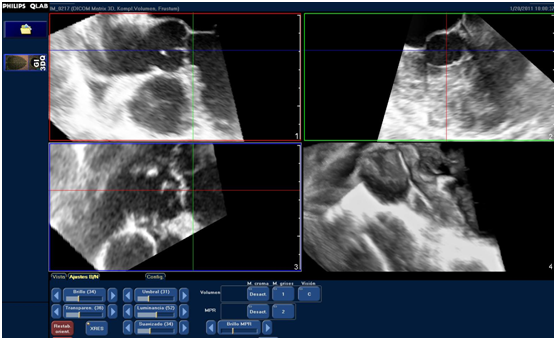
Figure 1 Acquisition of LVOT orthogonal planes: LVOT long axis can be observed (red and blue planes) and its orthogonal view at the short axis (green plane). Measurement of the larger and shorter diameters and of LVOT area are obtained by planimetry of the short axis from the green plane.
In general, as the systole advances, the septum protrudes towards the cavity decreasing the LVOT area. During protodiastole the smaller area of the LVOT was observed, coincidentally with certain septum persistence towards the cavity and the opening of the anterior mitral valve. Next, with the displacement of the anterior valve towards a partial closing position (mesodiastole), or partial opening (telediastole), the area increased again, reaching its maximun during the following protosystole. The LVOT total modification area was 17±12% (p<0.0001 between protosystole and protodiastole) (Table 2 & Figure 2).
|
|
Area (cm2) |
||
|
Phase |
Mean ± SD |
Median |
Ranges |
|
Protosystole |
4.36 ± 1.02 |
4.20 |
1.99-7.24 |
|
Mesosystole |
4.08 ± 0.94 |
3.95 |
2.06-7.14 |
|
Telesystole |
3.82 ± 0.93 |
3.77 |
2.20-6.96 |
|
Protodiastole |
3.59 ± 0.98 |
3.40 |
1.95-7.17 |
|
Mesodiastole |
3.77 ± 1.01 |
3.61 |
1.93-7.20 |
|
Telediastole |
4.15 ± 1.11 |
4.02 |
1.84-7.27 |
Table 2 Variation in LVOT area during the cardiac cycle
SD, Standard deviation
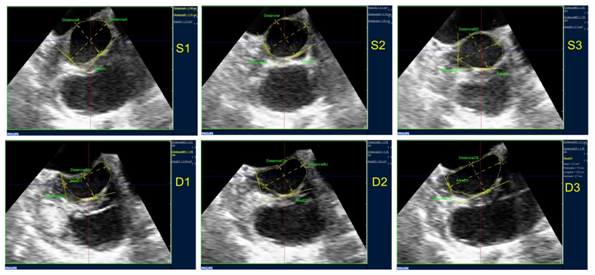
Figure 2 LVOT planimetry obtained in the different phases of the cardiac cycle (S1: protosystole, S2: mesosystole, S3: telesystole, D1: protodiastole, D2: mesodiastole, D3: telediastole). In this case the modification in area and shape (partially elliptic during the systolic phases, semilunar during the diastolic) can be observed.
In regards to the shape, it tended to be partially elliptic or circular during the systolic phases, and markedly elliptic or semilunar during the diastolic phases, the latter mainly due to the morphological and positional modifications of the anterior mitral valve at the LVOT: concave towards the LVOT during the systolic phases and plane or convex during the diastolic phases (Figure 2). The larger diameter at protosystole was 2.47±0.34 cm and the smaller 2.13±0.27 cm. The relation larger/smaller was 1.17 average at protosystole (a value near 1indicates a circular LVOT). Forty three percent of the patients had a larger/smaller diameter relation <1.1, compatible with a predominantly circular shape (coincident with visual estimate). The rest showed a partially elliptic shape, with the maximun larger/smaller diameter relation of 1.84 during this phase.
The larger diameter at protodiastole was 2.43±0.30 cm and the smaller 1.70±0.29 cm, with an average larger/smaller diameter of 1.46. Only two patients had a relation < 1.1. (Tending to circular shape), and the rest with elliptic or semilunar shapes depending on the degree of protrusion of the anterior valve during diastole (the maximun relation of larger/smaller diameters was 2.29). The increased variation of the LVOT was due to a modification in the smaller diameter, mainly caused by the movement of the anterior valve, with a difference between protosystole and protodiastole of 0.43±0.19 cm (percentage of reduction 20±9%, p<0.0001). On the other hand, the larger diameter had little variation (0.04±0.24 cm), without statistical significance (percentage of reduction 0.01±9%, p=0.99). Based on these findings, it was observed that the main contribution to the LVOT area and shape modification is carried out at the expense of the smaller axis and it gives the impression of depending more on the mitral component (anterior valve) than on the interventricular septum.
Presently, the estimation of the LVOT area is performed with diagnostic methods by bi dimensional images, through the formula π x (D/2)2, where D is LVOT diameter measured at the long axis at mesosystole.1 The implementation of new techniques for valvular disease therapy, such as percutaneous aortic valve replacement, requires a detailed evaluation of LVOT anatomy and the aortic root. The new 3D imaging methods allow visualizing that the normal LVOT anatomy is not circular in all cases as was assumed with bi dimensional methods. We can add that not always the bi dimensional cut passes through the central LVOT axis which is another source of potential error.2 At present, different studies have confirmed that LVOT anatomy is more elliptic than circular; a significant decrease of the estimated LVOT area related to the area measured by planimetry3–6 is also observable. This contributes to underestimate both larger and smaller LVOT diameters, which are essential for the correct choice of percutaneous prosthesis, increasing the risk of perivalvular leaks, and prosthesis migration, among other complications.7
In our study, we were able to verify the predominantly elliptic shape of LVOT, that was described thoroughly earlier, learning that this anatomic region is subjected to substantial changes, both in size and shape, during the cardiac cycle. Tops et al assessed the modification of the larger and smaller diameters via Multislice Cardiac Tomography (MSCT), verifying the elliptic shapes of LVOT, assessing in parallel its modification at telediastole and telesystole, without finding any significant difference in the diameter, using a regular sagittal oblique view, which usually corresponds with the left parasternal long axis view (minor axis).8 One of the limitations of this method is the low temporal resolution compared to the 3D TEE echo, with a frame rate no larger than 10-12 fps.
Although MSCT is one of the reference methods to assess the geometry of the aortic root, usually LVOT is frequently evaluated with echocardiography. Otani et al.,5 have reported a very good correlation between these two methods to assess the planimetric area of the outflow tract (r=0, 96 mean diference: 0.35 ± 0.29 cm2). This method would also allow modifying the continuity formula regularly used in the estimation of the aortic valve area (AVA). It is usually considered that AVA measurement using continuity equation over-estimates the severity of the aortic stenosis.9–11 This could be partly because the area is estimated and not directly measured, underestimating the numerator factor of the equation. Using the planimetric LVOT area measurement, Burgstahler et al.,4 evaluated the LVOT area and the AVA by planimetry through IMR in a population that showed normal and moderate to severe aortic stenosis. They showed that using the modified continuity equation with LVOT planimetric area resulted in better correlation with planimetric AVA measured with IMR.
The assessment of LVOT area modifications during the cardiac cycle found in the found in our study, opens a potential niche of interest on the interaction of the new devices employed in percutaneous aortic valve replacement. Particularly some devices that rest deeply on the whole LVOT, and its shape and modification during the cardiac cycle could influence its performance.
One of the limitations of this study is the absence of a gold standard in LVOT area measurement. Although correlation with MSCT is very good, the method has not been compared here. Patients were enrolled consecutively, but the number of patients with moderate and/or severe aortic stenosis was not high enough to allow comparison with the total cohort. Finally, patients with structural heart disease which modified LVOT dynamics were excluded; therefore these results cannot be extrapolated to those populations.
LVOT undergoes changes in its size and shape during the cardiac cycle, essentially at the expense of the minor axis due to the movement of the anterior mitral valve, and to a smaller degree of the interventricular septum. The 3D TEE echo emerges as a valid alternative in the evaluation of LVOT anatomy and geometry.
None.
The authors state that there is no conflict of interest.
None.

©2015 Falconi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.