Journal of
eISSN: 2373-4396


Case Report Volume 2 Issue 4
1Pediatric Cardiology Fellow, USA
2Clinical Cardiologist, USA
3Electrophysiologist Cardiologist, USA
4Pediatrician Cardiologist, USA
5Radiologist, Fundaci
Correspondence: Mosquera Walter, Adult and Pediatric Cardiology Unit, Fundación Valle del Lili, Cali, Colombia, USA
Received: March 14, 2015 | Published: April 9, 2015
Citation: Rocío V, Juan VC, Pablo P, et al. Non-compacted myocardium (ncm) with pre-exitacion syndrome: report of two cases. J Cardiol Curr Res. 2015;2(4):119-122. DOI: 10.15406/jccr.2015.02.00067
Non-compaction cardiomyopathy (spongiform cardiomyopathy) is a primary disorder of the ventricular muscle of unknown cause, which can occur at any age, often between 20 to 40 years of age. It affects mainly men, and can be asymptomatic or present with symptoms of heart failure, arrhythmias or embolic events. Color Doppler echocardiography and nuclear magnetic resonance (cMRI) are the most commonly used diagnostic methods. No specific treatment is currently available, and it is treated similarly to patients with heart failure. We report two cases of con compacted myocardium with pre-excitation syndrome.
Keywords:non-compaction cardiomyopathy, heart failure and diagnosis
Nineteen (19) years old Colombian male,who consulted the cardiology service, for a 7 years history of Grade II NHYA dyspnea, syncope in the last 4 years, and more recently intermittent chest palpitations, accompanied by atypical chest pain. The patient underwent surgical closure of patent arterious ductus at 10 years of age, and ablation of a right septal posterior pathway associated with supraventricular tachycardia (SVT) with. WPW syndrome, at 16 years of age. There is a history of allergy to dipyrone and metoclopropamide.
Physical examination and EKG
On admission blood pressure was 110/60mm/Hg, heart rate 136 beats per minuteand respiratory rate 18 breaths per minute. No other abnormalities were found. FBC, renal function tests, blood glucose, coagulation and electrolytes were within normal limits. Natriuretic peptide was 154pg/ml (normal<100pg/ml). The EKG documented atrial fibrillation witha high ventricular response to 140 beats per minute.
Diagnosis and management
A transesophageal echocardiogram ruledout intracavitary thrombi. The left chambers were dilated with slow flow in the left atrium. The walls of the left ventricle had normal thickness with severe overall decreased contractility, and the endocardium was thickened and hyper refringent at the middle and basal thirds. The apex was also thickened with increased number of trabeculae and obliteration of the cavity. Left ventricular function was 20-25%. A a bicuspid aortic valve, with mildregurgitation was found, the right atrium was severely dilated and the right ventricle was normal in size but its walls were thickened and severely hypo kinetic.
Pulmonary arterysystolic pressure was 46 mmHg, with moderate tricuspid regurgitation. No pericardial effusion was seen, and the aorta was normal with atrial septal integrity. Heart MRI revealed an altered left ventricular morphological pattern characterized by the presence of multiple trabeculae forming a reticular pattern, especially affecting the distal half of the ventricle. The interventricular septum bulged from left to right, and there was mild hypokinesia of the middle third of the interventricular septum, mainly towards its anterior half. No areas of abnormal uptake were found in the ventricular walls. There was no pericardial effusion and no infiltration of the myocardium or areas of scarring. The findings were compatible with non-compacted myocardium (Figure 1 & 2). An electrophysiological study ruled out the presence of an accessory hidden pathway. Due to the presence of an atypical slow atrial flutter fast degenerating into atrial fibrillation, no ablation was performed.
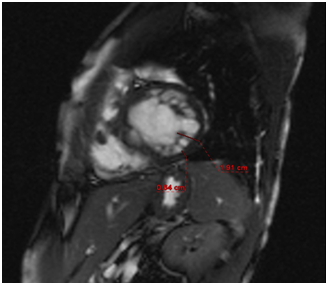
Figure 1 Altered relationship between the compact and the trabecular components of the ventricular wall (3.2 for normal <2.1 value at end diastole).
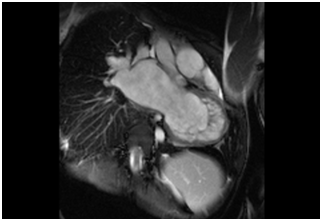
Figure 2 Long axis, bSSFP technique. The non compact component of the LV is observed. Increased size of the Left atrium is an associate finding.
Electrical cardio version was performed with conversion to sinus rhythm at 80 beats per minute. Due to the patient’s background and the severity of the left ventricular dysfunction, it was decided to implant an automatic defibrillator for prevention of sudden death (Figure 1).
Clinical presentation and background
This patient was a previously healthy 9 years old boy, NYHA functional class I. referred for pediatric cardiology assesment after incidental finding of a heart murmur. On examination a decreased ejection fraction was evident and the child had findings consistent with the diagnosis of ventricular non compaction. Denies episodes of syncope or arrhythmias. No chest pain. Perinatal or family histories were not relevant. Reported having suffered from dengue fever at the age of 5 years.
Physical examination and EKG
On examination weight was 28kg, and he was in good general condition, without signs of heart failure. Heart rate was 96 beats per minute, respiratory rate 22 bpm, blood pressure 100/63/75, and environmental oximetry 98%. No cyanosis was observed. Mobile neck without jugular venous ingurgitation, chest symmetrical without retractions, rhythmic heart sounds, presence of a grade II/VI (?) murmur in the left sternal region. EKG: sinus rhythm, 90 bpm with presence of short PR interval, negative aVR, positive aVL, positive in DI, DII, DIII, negative V1-V2, transition in V3.
Diagnosis and management
At the echocardiogram theleft ventricle was severly dialated, with decreased segmental contractility but its walls were normal in thickness. Diastolic function was normal. Multiple trabeculae were seen in the lateral, posterior and septal walls. Relationship compact/noncompact was 2.5:1. The basal septum was very dysknetic, and the septum as a whole moved paradoxically. The right ventricle was not dilated and without obstructions and its walls had normal thickness, and exhibited good contractility.
There was a mild regurgitation at the Tricuspidvalve; estimated pressure within the right ventricle of 22 mmHg. Mitral valve without regurgitation, E/A 1.7: 1 mitral DT 85. Mitral annulus 26 mm. No pericardial effusion. Other cardiac structures remained unaltered. The ejection fractionof 34% reported by the MRIC, together with the left ventricule trabecular pattern and the trabecular portion to compact portion ratio were, compatible with the echocardiographic findings suggesting Non-compaction cardiomyopathy. Other findings were: No evidence of intracavitary thrombi, left ventricular systolic dysfunction with increased ventricular volumes, dinterventricular septum dyskinesia, without highlighting areas suggesting the presence of macroscopic fibrosis or infiltrative at this level.
A Ten (10) minutes Cardiac Stress test according to BRUCE protocol, approached 12 METS. The resting heart rate was 84 bpm with maximum of 181bpm, which represents 85% ofthe maximum heart rate anticipated for the patient´sage. Resting blood pressure was 110/70mmHg and the maximum value reached 130/70 mmHg. The resting EKG pattern evidenceda Wolf Parkinson White syndrome and left ventricular hypertrophy. Functional capacity I. The cardiac frecuency in response to maximum effort, and the blood pressure in response to normal stress were normal. No chest pain was experienced. The report from Electrophysiology confirms a WPWR pattern,possibly of the right pathway. Pharmacological management was started with diuretics, ACE inhibitors and antiplatelet treatment.
A decision was initially taken to proceed with ablation of the accessory pathways. The presence of atrue accessory parahisiana pathway wasevidenced by the induction of an AV reentry arrhythmia, 150bpm 4 beats and through the mechanical ablation of the accessory pathway. With the positioning of the catheter. The patient remained in complete AV block for 10 minutes, with subsequent recovery of AV conduction, first by the bundle and subsequently by the accessory pathway. Gradually decreasing atrial pacing was performed without inducing arrhythmias, with a refractory period of the accessory pathway to 290bpm, and presence of second degree AV block. This was followed by to ventricular pacing, up to 3 additional stimuli, without induction of ventricular arrhythmias or AV reentry: Given the presence of a parahisian pathway, with evidence of AV conduction disorder and a high risk of complete AV block in an asymptomatic patient it was decided not to ablate.
At follow-up, the patient remains NYHA functional class I. A screening echocardiogram a performed to the sister did not reveal findings of compact myocardium. The patient was adviced to continue medications and doing mild exercise.
Isolated noncompaction cardiomyopathy its typical echocardiographic appearance were first described in1984 by Engberding and Bender in 1984, who identified this disorder as "isolated persistent myocardial sinusoids" due to a lack of normal regression during embryogenesis.1 In 1990, Chin et al suggested that the clinical picture should be called "isolated. Non-compaction cardiomyopathy of the left ventricle “based on a better understanding of the developmental physiology of the myocardium.2 This disorder can become manifest during childhood or adulthood. A 94 year sold male patient of with a transient ischemic cerebral attack was reported to have this disease.3 The diagnosis is more common among those aged 20-40 years,4 prevalence is higher in men than in women (2:1). It is a very rare disease, (incidence of 0.05% per year)5 and it can occur in isolation, although there have been reports of familial clustering in up to 44% of cases.6 Mortality 6 years after diagnosis is 80%, half of these cases is due sudden death.7 The right ventricle is compromised in less than 50% of patients. The cause for the lack of ventricular compaction is not known. One etiopathogenic mechanism proposed is an interruption of the embryonic myocardial compaction process between the fifth and eighth weeks of gestation.8 Among the cardiac abnormalities associated with non-compact cardiomyopathy is not compacted myocardium with sinusoids and fistulae of the right coronary artery, congenital anomalies in the outflow tract of the right and left ventricle,9-13 Ebstein's anomaly, bicuspid aortic valve and transposition of great arteries, ventricular septal defect, besides metabolic and genetic disorders such as Barth syndrome abnormalities, Charcot-Marie-Tooth and Melnick-Needles syndrome.14 Most patients have nonspecific electrocardiographic abnormalities such as, left ventricular hypertrophy, repolarization disorders, inversion of the T wave, ST segment changes, intraventricular conduction disorders and heart blocks.15 Atrial fibrillation has been reported in over 25% of adults with NCM and ventricular tachyarrhythmia in up to 47% of patients. The association with bradycardia and Wolf Parkinson White syndrome In pediatric patients is about 18%.16,17 All ll studies in pediatric patients with NCM have shown higher prevalence of familiar cases, facial dysmorphisms and Wolf Parkinson White syndrome compared with adults in whom the usual findings are secondary arrhythmias,18-22 as well as more cardiovascular symptoms. NCM may be asymptomatic or it can present with symptoms of severe left ventricular dysfunction, arrhythmias and embolic events.23-25 In a small caseseries, mean time to onset of symptoms after diagnosis was 3 years and 6 months.5 More than 2/3 of adult patients with symptomatic heart failure had NCM.2 In another study with equally small numbers the clinical manifestations were dyspnea in 79% of patients, functional class III-IV heart failure in 35% of patients, chest pain in 26%, and atrial fibrillation in 26%.19
Frequency of thrombo embolism range from 21% to 38% and may be the result of depressed ventricular function, atrial fibrillation or may be due to thrombus formation in the trabeculae.18 Cardiac catheterization in children may reveal a restrictive hemodynamic pattern as the initial presentation of isolated NCM.19,23 In a Japanese cohort study of pediatric patients followed for 17 years, most patients developed ventricular dysfunction, independently of whether or not they were symptomatic at onset. In review studies with more patients, Wolf Parkinson White syndrome develops in only 7% of patients, and is more prevalent in children.23 Diastolic dysfunction t in NCM may be due to an abnormal relaxation and restrictive filling caused by the numerous prominent trabeculae20 found mainly in the apex and in the lateral and inferiorwall of left ventricle21 the apex is always the most compromised.23
Ischemic sub-endocardial lesionshave been found postmortem, which has given importance to the theory that there are abnormalities in the microcirculation that may play a role in the pathophysiology of the condition. PET imaging has shown that there is a decrease in reserve coronary flow, both in noncompacted and compacted areas.22
The diagnosis of NCM is made with the help of two-dimensional color Doppler echocardiography or magnetic resonance imaging MRI, which has become the method of choice to confirm or exclude NCM. There are different echocardiograph criteria, of which the most frequently used are those proposed by Jenni et al., referring to the absence of coexisting cardiac abnormalities, segmental thickening of the left ventricular myocardial wall with 2 laminae: a thin epicardial lamina and a thick endocardial lamina with prominent trabeculae and deep recesses. This is the relationship between the compact and non-compact myocardium at the end-of) the location of the trabeculae in the apical/lateral, middle/lower walls of the left ventricle, where most of the segments of the uncompacted heart do not contract properly. Color Doppler can identify flow among the intertrabecular recesses.23 For MRI the diagnostic criterionis that of Petersen et al. This is the ratio between the compact and noncompact portion of the myocardium greater than 2.3 measured at the end ofdiastole, which is usually bigger than 2,3.25,26
Also used are Jaquer’s et al.25 criteria which consider the trabecular left ventricular mass, which should be greater than 20% of the overall mass of the same ventricle measured at end of diastole. Other diagnostic aids are CT and ventriculography. Among the differential diagnoses of NCM, it is mandatory to exclude endomyocardial fibrosis, infiltrative cardiomyopathy, dilated cardiomyopathy, hypertensive heart disease and apical hypertrophic cardiomyopathy.
Currently there are no specific treatments for this disease. As in the standard management for heart failure, beta blockers, ACE inhibitors should be used with the aim of reducing the morbidity and mortality of this disease.26 Arrhythmias are treated with beta blockers, calcium channel blockers or amiodarone, as appropriate. Carvedilol has demonstrated beneficial effects in left ventricular function.25 For the prevention of embolic complications several authors recommend anticoagulation in all the patients. However, the general idea is to give oral anticoagulants only for patients who have had a thromboembolic event or have a condition such as atrial fibrillation.
Based on a study of 36 children followed at the Children's Hospital of Texas, where no cases of thromboembolism were detected, for children Pignatelli et al. recommend the use of antiplatelet agents such as aspirin. The biventricular pacemaker can be part of the management of patients with heart failure, ventricular dysfunction and intraventricular conduction disorder; likewise sudden deathmust be prevented with the use of implantable cardioverter defibrillators, especially in patients affected by with serious arrhythmias such as ventricular tachycardia.26 Some patients with heart failure refractory to medical treatment have had cardiac transplantations.24
The prognosis is still unclear. However, it has been observed in both children and adults that prognosis is better if the diagnosis is made while the patient is still asymptomatic. In adults prognosis is poor in patients with heart failure stages III and IV, those with ventricular diastolic dysfunction, left bundle branch block and atrial fibrillation. Since up to 50% die of sudden death, monitoring and patient management should be strict. Given that most genetic associations are found in children, it is highly recommended to study the family members.26
The non-compaction cardiomyopathy remains a difficult disease diagnosis. It requires a high index of suspicion to do so. In our cases we observed that the diagnosis was done late, with the risk of sudden death that is documented in some patients. We note the variability in the clinical presentation of our patients.
None.
There is no conflict of interest.
None.

©2015 Rocío, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.