Journal of
eISSN: 2469 - 2786


Research Article Volume 6 Issue 1
Eman H Abed, Environment and water Directorate, Ministry of Science and Technology, Baghdad, Iraq
Correspondence: Eman H Abed, Environment and water Directorate, Ministry of Science and Technology, Baghdad, Iraq
Received: January 09, 2017 | Published: January 17, 2018
Citation: Jawad MM, Abed EH, Oudah HK. Using of Ceratophyllum demersum l.for lead and cadmium pollution removal by columns technology. J Bacteriol Mycol Open Access. 2018;6(1):19-21. DOI: 10.15406/jbmoa.2018.06.00169
Environmental exposure to toxic heavy metals is one of the main critical issues on environmental and public health. Heavy metals are common pollutants in aquatic ecosystems, which are particularly susceptible and often final receptor of heavy metals. Phytoremediation with aquatic plants is a new, effective and inexpensive method for improving water quality and wastewater. In this study, Lead and Cadmium (50ppm) of industrial water polluted were removed by the aquatic plant, Ceratophyllum demersum L., as grinded and crushed. Results showed that grinded plant was the best in removing Lead and Cadmium than crushed plant, so, removing concentration of Lead by Ceratophyllum demersum L. grinded and crushed were (38, 37.8, 37.5, 33.1 and 30.8), (23, 22.5, 22.5, 18.4 and 12.2) ppm and removal percentage (76, 75.6, 75, 66.2 and 61.6), (46, 45, 45, 36.8 and 24.4) % at flow rate (5, 10, 15, 20 and 25) ml/min respectively. Whereas, removing concentration of Cadmium were (29.5, 29.5, 30, 22.5 and 14.4), (13, 12.5, 12.5, 8.4 and 2.2) ppm and removal percentage (59, 59, 60, 45 and 28.8), (26, 25, 25, 16.8 and 4.4) % at flow rate (5, 10, 15, 20 and 25) ml/min respectively.
Keywords: ceratophyllum demersum, lead, cadmium, water pollution
Environmental pollutant and its harmful effect on ecology have been studied intensively during the last decades. The removing of pollutants from wastewater was increased with the fast industrial development. These wastewaters are produced in large amounts and must be treated before discharge.1
Heavy metals are very harmful for humans, animals and plants. Global and local agencies have therefore established certain limits on the quantities of heavy metals being that discharged into the environment. The most widely used methods for removing heavy metals are chemical or electrochemical precipitation, both of which pose a significant problem in terms of disposal of the precipitated wastes.2
When heavy metals are could be high accumulate in living tissues, Cadmium, lead and copper can become a sanitary and ecological threat to drinking water resources, even at very low concentrations. Cadmium and zinc are common industrial pollutants, as well as their harmful effect to plant at relatively low concentrations.3 Thus, there was a need to use cleaner alternatives must be developed in order to remove heavy metals from effluents.4 Occurrence of water polluted with toxic metals in plants and human being water bodies adversely affects the lives of local people since they utilize this water for daily requirements. The heavy metals can be incorporated into the food chain and their levels can increase through biological magnification.5
Studies indicate that there are many plants that can drag and accumulate ofheavy metals from contaminated areas, but the ideal plant for this process should be as specific features available, such as the pace of growth and roots, mass and ease of harvesting and cutting and accumulation of a wide range of elements, in addition to carry around high levels of these elements.6
Lead (Pb), cadmium (Cd), cobalt (Co), chromium (Cr) and mercury (Hg), which have the toxic effects of high concentration as well as low concentrations of plants. Lead is accounted significant pollutant due to solubility in water, which results in wide distribution in the aquatic ecosystems lead, is strongly toxic to organisms. The excessive amounts of lead in water cause many physiological and biochemical stress symptoms in plants, such as growth reduction, disturbed mineral nutrition, water imbalance and growth productivity and root elongation. When they enter inside the cell wall like any other heavy metals they produce an oxidative stress in plant and lead to cell damage.7 The aim of this study was to use Ceratophyllum demersum and its ability on wastewater for recycling to reuse for other purposes in agriculture and industrial fields.
Plant collection
Samples of the plant C. demersum L. were collected from existing channels at Baghdad University, during April and May 2017. Then the plant dried by using oven at 65°C for three days, then breathing and grinding. The plant filled in a glass column prepared for this purpose in the laboratory.
Adsorption column preparation
Glass columns used with a diameter (7 cm) and (50 cm) length filled with grinded and crushed C. dermersum plant. Filter paper was put at the end and the beginning of each column. The two columns are joined with container (5) liter capacity of each, in order to feed the column with polluted water with (Lead and Cadmium) at a flow rate (15ml/min) of first container while the 2nd for aggregation water after recycle in column at approximately (5,5hrs) impairment time.
The biological treatment system design(designed bioremediation system):
The form of (Figure 1), which were established in the laboratory for the treatment of water contaminated with heavy metals using a glass column container for each of them grinded and crushed C. demersum L separately. The system is composed of:
Removal of lead by grinded and crushed of C. demersum L:
Removal of lead element with concentration (50 ppm) by grinded and crushed plant C. demersum L. from polluted water by using adsorbent column with different flow rate ranged (5, 10, 15, 20 and 25) ml/min. Results shown that the best removal of a vital element for lead by using grinded C. demersum L. which was obtained at flow rate (5, 10 and 15) ml/min, with percentage of removal (76, 75.5 and 75) %, respectively, whereas, the crushed plant was a decent efficiency in removal the Lead with (46, 45, 45, 36.8 and 24.4) % at flow rate (5, 10, 15, 20 and 25) ml/min, respectively (Table 1) (Figure 2). Phytoremediation, a method to remove pollutants from the environment by using plants and algae, has been known as a promising cost-effective and environmentally sustainable technology for the remediation of water polluted by toxic trace elements.
Con. of Lead (ppm) after Treating |
Removing Con. of Lead (ppm) |
Removal % |
Flow Rate |
|||
Grinded |
Crushed |
Grinded |
Crushed |
Grinded |
Crushed |
|
12 |
27 |
38 |
23 |
76 |
46 |
5 |
12.2 |
27.5 |
37.8 |
22.5 |
75.6 |
45 |
10 |
12.5 |
27.5 |
37.5 |
22.5 |
75 |
45 |
15 |
16.9 |
31.6 |
33.1 |
18.4 |
66.2 |
36.8 |
20 |
19.2 |
37.8 |
30.8 |
12.2 |
61.6 |
24.4 |
25 |
Table 1 Clinical and biochemical variables of individuals with overweight-obesity
SD: Standard Deviation; BMI: Body Mass Index; WC: Waist Circumference; AC: Abdominal Circumference; HC: Hip Circumference; RER: Respiratory Exchange Ratio; HR: Hear Rate.
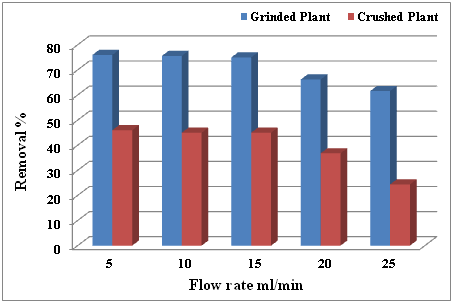
Figure 2 Removal percentage of Lead (50 ppm) by grinded and crushed of C. demersum L. at different flow rate.
Remove of cadmium by C. demersum L. grinded and crushed column:
The polluted water with Cadmium element (50ppm) in adsorption experiments column of grinded and crushed C. demersum L plant was removed by grinded plant with (59, 59 and 60) % at flow rate (5, 10 and 15) ml/min respectively, whereas, the crushed plant was a decent efficiency in removal the Cadmium with (26, 25, 25, 16.8 and 4.4) % at flow rate (5, 10, 15, 20 and 25) ml/min, respectively, (Table 2) (Figure 3), and the best flow rate in removing Lead and Cadmium by grinded and crushed plant C. demersum L. were (5, 10 and 15) ml/min.
Con. of Cadmium (ppm) After Treating |
Removing Con. of Cadmium (ppm) |
Removal % |
Flow Rate |
|||
Grinded |
Crushed |
Grinded |
Crushed |
Grinded |
Crushed |
|
20.5 |
37 |
29.5 |
13 |
59 |
26 |
5 |
20.5 |
37.5 |
29.5 |
12.5 |
59 |
25 |
10 |
20 |
37.5 |
30 |
12.5 |
60 |
25 |
15 |
27.5 |
41.6 |
22.5 |
8.4 |
45 |
16.8 |
20 |
35.6 |
47.8 |
14.4 |
2.2 |
28.8 |
4.4 |
25 |
Table 2 Removal of Cadmium (50ppm) using packaged treatment C. demersum L. grinded and crushed at different flow rates
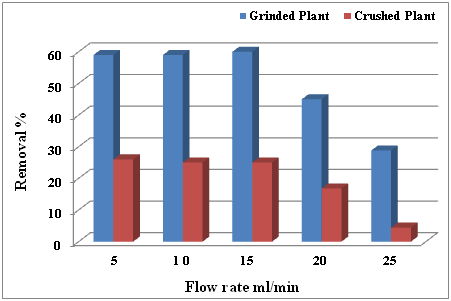
Figure 3 Removal percentage of Cadmium (50 ppm) by grinded and crushed of C. demersum L. at different flow rate.
The C. demersum L. plant has the ability to absorb and accumulate a large amount of Lead and Cadmium, which makes it useful as indicators of biological weapons. It has proven its ability cumulative Cadmium in contaminated water up to 1000ppm.8 Index bio indicator for this type of plant on the viability of deals in reducing water pollution with heavy metals.9 Some environmental scientists improve that this type of aquatic plants (C. demersum L.) have the ability to remove the bullets Lead, Nickel and Cadmium more than the rest of Iron, Manganese and Zinc.10 Studies have shown that the greatest potential to alleviate damage metals from sewage that address activities by absorption and desorption of surface water plants.11,12 Also dangerous element Cadmium is not limited to small organisms even on humans.13,14 The plant water C. demersum L. has the ability to adsorption and carrying toxic heavy metals.15 From these results observed that the percentage removal of Lead from the solution when used aquatic plants C. demersum are high. These results are in accordance with Majid and Siddique.16,17
We conclude from this study on the ability of aquatic plants to remove some heavy elements from industrial water that cause environmental pollution. The grinded of C. demersum L. plant proved to be efficient in removal of Lead and Cadmium than crushed plant, when packed in a glass column with a flow rate of (5, 10 and 15) ml/min.
None.
The author declares no conflict of interest.

©2018 Jawad, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.