Journal of
eISSN: 2469 - 2786


Research Article Volume 4 Issue 3
Department of Biosciences, College of Natural and Applied Sciences, Salem University Lokoja, Nigeria
Correspondence: Ekpa Emmanuel, Department of Biosciences, College of Natural and Applied Sciences, Salem University Lokoja , Nigeria, Tel 2.73704E+11
Received: November 03, 2016 | Published: March 16, 2017
Citation: Emmanuel E, Andrew A, John OC, et al. Isolation, identification and characterization of some bacteria from soil samples of agbaja iron ore mining site of kogi state. J Bacteriol Mycol Open Access. 2017;4(3):79-84. DOI: 10.15406/jbmoa.2017.04.00092
Hydrometallurgical and other conventional mining technologies on low grade ores require high energy and capital costs. The use of microbes to degrade such type of minerals is gaining acceptance. This study was carried out, therefore to isolate, identify and characterize iron solubilizing bacteria from Iron stones of Agbaja iron ore mining site of Kogi State. Crushed samples in the range of 0.25µm and 0.75µm particle size were used for the isolation. They were cultured in a modified 9k media to facilitate bacterial growth and pure cultures were then isolated and sub-cultured for further identification. Morphological and biochemical analysis suggests that some of the bacteria identified are members of Acidithiobacillus, Pseudomonas, and Leptospirillum species. Also results of growth pattern due to bacteria count obtained after 24hours, 48hours and 72hours of incubation ranged between 0.1×103cfu/ml and 12.3×103cfu/ml. The highest count of 12.3×103cfu/ml was obtained in Pit 4 at 72hours. At 24hours there was no visible count in Pit 5 and 4, while at 48hours pit 4 was 1×103 and pit 5 was 2.6×103 and finally at 72hours pit 4 and 5 was 12.3×103 and 4.3×103 respectively, incubation time, and temperature, further confirms that these isolated species are mesophilic-acidophiles with good bioleaching properties.
Keywords: iron ore, 9k media, bioleaching, bacteria
An ore is a type of rock that contains sufficient minerals with important elements including metals that can be economically extracted from it.1 The ores are extracted through mining. The ores are usually rich in iron oxides and vary in colour from dark grey, bright yellow, deep purple, to rusty red. Biomining is a general term used to describe the use of microorganisms to facilitate the extraction of metals from sulfide or iron-containing ores or concentrates.2 Bemoaning encompasses two related microbial processes that are useful in extractive metallurgy: bacterial leaching, also known as bioleaching, and bio-oxidation. Sulfides and oxides of metals such as zinc, copper, nickel, and cobalt are almost insoluble in water, but the sulfates of these metals are readily soluble. When the metal sulphide is oxidized to its sulphate, the metal is leached into solution from where it can be extracted.
Bioleaching involves the use of microorganisms to catalyze the oxidation of iron sulfides to create ferric sulphate and sulfuric acid. Ferric sulfate, which is a powerful oxidizing agent, then oxidizes the metal sulfides minerals, which are given above and the metal contained is then leached by the sulfuric acid formed. Current understanding of the mechanism of solubilization is that it is primarily a chemical process, although attachment of microbes to the mineral can enhance dissolution.3 This strategy for metal recovery is known as bioleaching because the metal is solubilized in the process. On the other hand, bio-oxidation is an oxidation process caused by the microbes where the valuable metal remains in the solid phase. Likewise, in coal desulfurization, bacteria are used to oxidize the pyrite contaminant in the coal thus making the sulfur soluble as ferric sulfate.4 Worldwide reserves of high-grade ores are diminishing at an alarming rate due to the rapid increase in the demand for metals.5 World copper production has increased steadily in the period 1984- 2005, from 9 metric ton to 16 metric ton per annum. Likewise, world gold production has risen from 1187 tonne to 2471 tone in the period of 1980-2000.6 As higher grade mineral deposits become worked out, there is an increasing need to recover metals from lower-grade mineral deposits.
Bacterial based methods have clear economic advantages in the extraction of metals from many low-grade deposits. Other benefits of the process are its relative simplicity, mild operation conditions, low capital and operating costs, low energy input, and being more environmentally friendly than physical-chemical processes.7 Specified another advantage of the process that tailings from bio-mining operations are less chemically active and the biological activity they can support is reduced by the extent to which they have already been bioleached. Mine tailings and wastes produced from physicochemical processes might be biologically leached when exposed to rain and air, producing unwanted acid and metal pollution. The need to mitigate environmental pollution due to discharges from mining activities calls for more studies. The interactions between microorganisms and iron stones needs further investigation, hence the need for this present work. Molecular characterization of the genes responsible for bioleaching capabilities of bacteria begins with isolation and identification of pure cultures. Literature abound with different types of bacteria isolated from various mining sites around the world, there is need therefore to study those from Agbaja. Due to the diversity of micro organisms found within mining sites as reported in different literature, this work was therefore set out; to isolate and identify the various types of bacteria inhabiting such mining sites and to determine the relationship between them and their environment.
Materials
All reagents and chemicals used in this study were of high analytical grade. Some of the equipment used are include Intelligent Thermostatic Shake Cultivation Cabinet (incubator shaker) made in England, Vertical Heating Pressure Steam Sterilizer (autoclave),Colony Counter, General Laboratory Oven made in and Laboratory Incubator.
Sample collection
Crushed iron ore samples were collected at different location within Agbaja iron ore mining site from upper layer where most of the microbial activity takes place and thus where most of the bacteria population is concentrated. Iron ore samples were collected using some clean dry and sterile polythene bag along with sterile spatula. 50g of the iron ore samples were dissolving in 100ml of distilled water to make iron ore suspensions and kept on an incubator shaker for 7days.
Isolation of iron ore bacteria
50g of each sample of Agbaja iron ore sample was introduced into a conical flask containing 100ml of distilled water. The pH was adjusted to between 2.0-5.0 using 0.1ml of concentrated sulphuric acid (H2SO4). The conical flasks were plugged with cotton wool. Thereafter the suspension was homogenized and incubated for 7days in the incubator shaker at 30°C.
Media preparation
A 9K modified media were used. They contain the following composition; C6H12O6 (25.00g), CO(NH2)2 (2.00g), K2HPO4 (0.25g), MgSO4.7H2O (0.0625g). These media were used in order to get colonies of bacteria. The media was autoclaved at a temperature of 121°C for 15minutes. It was allowed to cool, 9ml of the diluted sample stored previously (in the incubator shaker) was pipette into sterilize petrish dish. The cooled media was also dispensed into Petri dish and allowed to gel, and incubated for 24hours at 37oC. They were cultured in a modified 9K media to facilitate and maintain bacteria growth.
Sample inoculation
Portions of the suspension were inoculated on the nutrient agar by streaking and were incubated at 37°C for 24hours. Then the bacteria strains were isolated from iron ore samples.
Examinations of purified bacteria cultures
In order to identify the purified cultures tentatively on the gram staining and various biochemical tests were performed namely; catalase test, oxidase test, and growth in NaCl test, etc.
Morphology
The colony morphology was viewed with the aid of a colony counter. Morphological characteristics such as colony, edges, surface, and elevation were observed.
Gram staining 8
A drop of distilled water was placed on a clean glass slide and a loop of the bacterial isolate was smeared on the water which was then allowed to dry. It was then passed through a flame twice in order to heat fix it. One to two drops of crystal violet was added and left for 60seconds before washing it with distilled water. Grams iodine was added and washed away with distilled water after 1minute. It was then flooded with alcohol (ethanol) and washed with distilled water after 30seconds and then adding safranin which was also washed with distilled water after 30seconds. The glass slide was left to dry and examined under the microscope.
Catalase test 8
This is a test to ascertain the ability to produce Catalase that reduces hydrogen peroxide to water and oxygen. On a clean glass slide, a smear of the organisms was made in a drop of normal saline with a flame sterilized wire loop. Thereafter, a drop of hydrogen peroxide was placed on the smeared organism. If effervescence occurs, it is confirmatory positive test for catalase production, but if it does not occur it is negative test for catalase production.
Oxidase test8
A piece of filter paper was soaked in oxidase reagent and the paper placed in a clean sterilized petri-dish. With an inoculating loop, a smear of the organism was made in a drop of normal saline on the filter paper and left for 30seconds. Purple colour shows a positive test, colorless is negative.
Growth in NaCl
This is a test to ascertain the ability of the isolate to tolerate high salt concentration. About (7g) of NaCl was dissolved in 100ml (7% solution) of Nutrient agar and was poured into slant bottles. The isolate was inoculated into the medium; incubation was for 24hours at room temperature.
Total bacteria count
The total bacteria count for the Ore samples are shown in (Figures 1 & 2). The result obtained after 24hours, 48hours and 72hours of incubation ranged between 0×103cfu/ml and 12.3 ×103cfu/ml. The highest count of 12.3 ×103cfu/ml was obtained in Pit 4 at 72hours. At 24hours there was no visible count in Pit 5 and 4, while at 48hours pit 4 was 1×103 and pit 5 was 2.6×103 and finally at 72hours pit 4 and 5 was 12.3×103 and 4.3×103 respectively.

Plate 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.

Plate 2 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
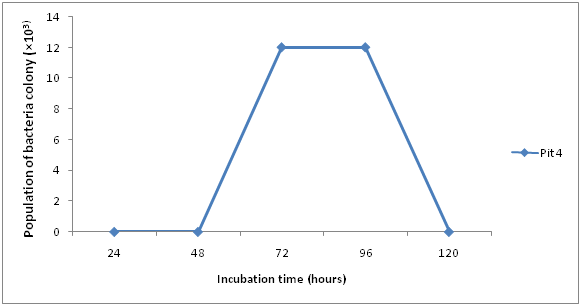
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
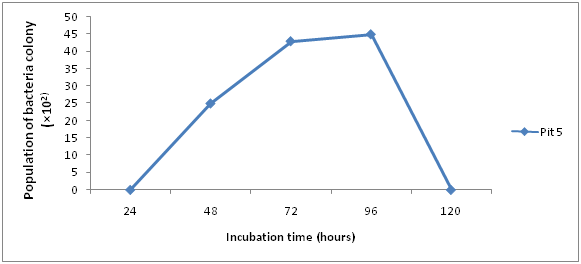
Figure 2 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
Growth rate of bacteria
The growth rate of bacteria present in iron ore sample are shown in (Figures 3 & 4), Pit 4 had a peak growth rate of 83% at 48hours, and least growth rate of 0 at 24hours. Pit 5 had a peak growth rate of 100% at 48hours and least at 24hours.
Results of Isolation, Identification and Characterization of bacteria from Agbaja iron ore mining site is shown in (Table 1 & Figures 1-5) respectively. A simple modified enrichment 9K media for the growth of iron oxidizing bacteria shows that it possess certain advantages than the conventional nutrient agar.9 Some of these advantages include: Easy preparation, Rapid growth of cultured organism and appearance of large visible colonies that are easily identifiable.10 Reported that normal nutrient agar inhibits the growth of iron oxidizing bacteria. The results of morphological and Biochemical Characterization (Table 1) shows that some of the bacteria belong to the Acidithiobacillus species and a few other Bacillus species that have been well documented in literature.11 Also results of growth pattern due to bacterial count, incubation time, and temperature (Figures 1-5) further confirms that these isolated species are mesophilic-acidophiles in agreement with.12
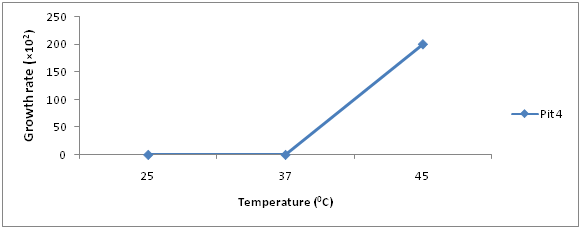
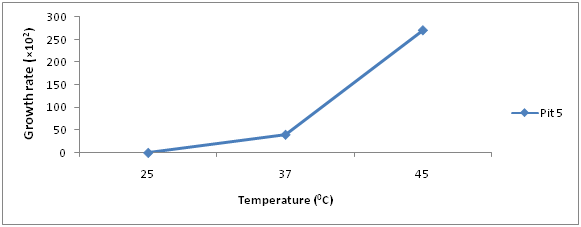
Figure 5 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
Isolates |
Form |
Colour |
Opacity |
Edges |
Elevation |
Cellular Morphology |
Gram Stain |
Oxidase |
Catalase |
NaCl |
Citrate |
Sucrose |
Glucose |
Fructose |
Urease |
Tentative Microorganisms |
1 |
Circular |
White |
Opaque |
Entire |
Flat |
Rod |
- |
- |
+ |
- |
+ |
A |
A |
A |
+ |
Acidithiobacillusspp |
2 |
Circular |
Cream |
Opaque |
Entire |
Flat |
Rod |
- |
- |
- |
- |
+ |
+ |
+ |
A |
- |
Leptospirallumspp |
3 |
Irregular |
White |
Opaque |
Lobate |
Raised |
Rod |
+ |
+ |
+ |
- |
+ |
+ |
A |
A |
+ |
Pseudomonas spp |
Table 1 Identification and Characterization of Isolates
Generally, metal mobilization from rocks, mineral soil and other substrates can be achieved by proteolysis, complication by excreted metabolites and iron (iii) binding via methylation.2 This result in volatilization. It has been shown that microbes can also mobilize metals and attack mineral surfaces by redox processes.13 For example, bacterial iron (iii) reduction results in release of some metals like Fe, Mn, and Co, from goethite ore (like those of Agbaja). Growth pattern observed in this studies shows optimum at 48hours and 37°C respectively. This might not be far from the fact that most Acidithiobacillus species of bacteria are mesophilicacidophiles.14 One can say that the optimum growth seen at 48hours is an indication of the log phase of the organisms presently availability of nutrients and less competition.
The frequency of the observed species of bacteria within this mining site may be attributed to the presence of Siderophore.15 These are the largest class of known compounds that can bind and transport or shuttle iron across microbial cells. They are excreted by a wide variety of bacteria (Bacillus species inclusive). They aid iron assimilating by these organisms.16 It has been established that iron is the only known essential element for which these specific organic shuttles operate. Organisms like the ones identified in this work have evolved mechanisms to ensure that iron demand is met through the production of species specific siderophores or by attachment to a solid iron mineral like goethite used for this work.13 Some bacteria of Acidithiobacillus species, Leptospirillum species, and Pseudomonas species (all identified in this work) have the ability to oxidize ferrous iron enzymatically with concomitant energy generation as reported by.17 This too may have informed the observed growth kinetics of the graphs (Figures 1-5). Iron (iii) may also be locally concentrated by adsorption to microbial surfaces and metal oxides. Microbial (bacterial) formation of hydrous iron oxides in aqueous environments may cause accumulation of other metal ions by co-precipitation or adsorption. Such adsorped metals may be remobilized by the reduction of the iron oxides or acidification.18
Microbes (bacteria and fungi) play a key geoactive roles in the biosphere, particularly in the ares of biotransformation, biogeochemical cycling, metal and mineral transformation, bioweathering and soil sediment formation. Microbes have a variety of properties that can effect changes in metal speciation, toxicity, and mobility, as well as mineral formation or dissolution.19-50 The ubiquity and importance of microbes in the biosphere make geomicrobiology one of the most important phenomenoum requiring an interdisciplinary approach to define environmental and biotechnology exploitation. From this work and the results observes in the choice of media growth kinetics and identifiable bacteria (Figures 1-5) it can be seen that microbes (bacteria) interacts with metals and minerals in both natural and synthetic environment. These alter both the physical and chemical state of this metals which aid microbial growth, activity, and survival. It can therefore be conducted that many minerals are biogenic origin, and formation of such biominerals of global geological and industrial significance.51-71
None.
The author declares no conflict of interest.

©2017 Emmanuel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.