Journal of
eISSN: 2469 - 2786


Research Article Volume 5 Issue 4
Department of Biotechnology, Cochin University of Science and Technology, India
Correspondence: Sarita G Bhat, Department of Biotechnology, Cochin University of Science and Technology,Cochin-682022, Kerala, India, Tel 91 484 2576267
Received: July 14, 2017 | Published: October 9, 2017
Citation: Sritha KS, Shemymol KA, Bhat SG. Infectivity criteria for use of ? sep2 and ? sep3 as bio-control agents. J Bacteriol Mycol Open Access. 2017;5(4):322-326. DOI: 10.15406/jbmoa.2017.05.00140
The emergence of multi-drug resistant Salmonella, one of the major pathogens causing food poisoning is challenging. Salmonella Enteritidis infections in poultry are widespread and the quest for safe alternatives to antibiotics led to phages. The purpose of this study was to isolate and characterize a novel pathogenic/lytic phages that targets Salmonella and to investigate its efficacy at lysing the bacterium. Poultry isolated Salmonella BT55CW showed considerable antibiotic resistance with MAR index of 0.4. Two lytic phages ɸSEP2 and ɸSEP3 were isolated using BT55CW Salmonella strain to control Salmonella infections. Morphology, host range and growth kinetics were studied. Influence of various physical and chemical factors such as pH, temperature, NaCl concentration and survival under different nutrient deprived conditions on the infectivity and persistence of phage forms the crux of the study. By TEM analysis ɸSEP2 and ɸSEP3 showed typical features of Podoviridae and Myoviridae respectively. Both lysed 60% all the Salmonella strains tested, survived at nutrition depleted conditions and substantially reduced Salmonella growth. Both phages showed considerable stability in the range of pH and temperature studied, but ɸSEP3 was less susceptible and hence a better candidate of the two as bio-control agent.
Keywords: salmonella-phage-bio control- gastroenteritis- antibiotic-resistance, ɸsep3, bt55cw salmonella strain
Salmonellosis caused by non-typhoidal Salmonella is a major public health concern as it is one of most common and frequent food-borne illness characterized by gastroenteritis, with an incubation period between 4 and 72 h.1 Non-typhoidal Salmonella causes around 93.8 million illnesses and 155,000 deaths each year worldwide, with 96% due to contaminated food.2 Contaminated poultry products are main vehicles of Salmonella infection. Frequent colonization of the poultry population with Salmonella causes the transmission to humans through meat and eggs.3 Abuse and uncontrolled use of antibiotic caused emergence of multidrug- resistant Salmonella ,4 which is alarming as it may result in a significant upsurge in morbidity and mortality. Concerted research to develop an alternative to antibiotics is the need of the hour and phages are a good option. Phages being natural antimicrobial agents against bacterial pathogens, have little or no harmful effect on human and animal immune system.5 Being species specific it enhances food safety6 and the Food and Drug Administration of the United States of America approved certain phages as “generally regarded as safe” (GRAS) for use in food products to control. This study therefore aimed to isolate, purify and characterize Salmonella phages to employ as bio control agent against Salmonella infections in poultry farms. Here comparison of phage characteristics for a better understanding of phage biology was intended, as environmental conditions influence phage stability, physiological state of bacteria and consequently host–phage interaction, as well the ability of the phage to reduce bacterial numbers. Detailed data on their morphology, growth parameters, stability studies, host range are described for bacteriophages specified for Salmonella species.
Bacterial isolation and identification
Salmonella BT55CW was isolated from chicken waste following the guidelines of the Bacteriological analytical manual, American food and drug administration (December 2015). Briefly, samples were inoculated in lactose D broth, incubated at 37 ̊C for 24 h, followed by inoculation into Rappaport-Vassiliadis (RV) and tetrathionate broth (TT) and incubation at 42 ̊C for 18 h and 37 ̊C for 24 h respectively. Loopful culture from RV and TT each were streaked onto Xylose Lysine Desoxycholate (XLD) as well as on Hekteon enteric agar (HE) and incubated at 37 ̊C for 24 h. Positive colonies were confirmed by biochemical tests and a portion of the 16S rRNA gene (1.5 kb) was amplified from the genomic DNA.7,8 The amplicon was sequenced and compared with the sequences in Genbank using BLAST.9 The partial sequence was deposited in the Genbank database (accession number KP992876).
Antibiotic sensitivity of isolate
The susceptibility test of strain BT55CW to different antibiotics was conducted as per Kirby-Bauer disc diffusion method10 with ten known antibiotics (Himedia, Mumbai, India) namely, ampicillin (5μg/disc), azithromycin (15μg/disc),cefixime(5μg/disc), cefuroxime (30μg/disc),chloramphenicol(30 μg/disc),ciprofloxacin (5μg/disc), gentamicin(10μg/disc), nalidixic acid (30μg/disc), tetracycline(30 μg/disc), trimethoprim (5μg/disc). MAR index was calculated.11
Bacteriophage isolation
Bacteriophages ɸSEP2 and ɸSEP3 were isolated using the direct method.12 The presence of bacteriophages in the filtrate was confirmed by double agar overlay method.13 For large scale production, phages were concentrated using Polyethylene glycol (PEG) 600014 and phage titer was determined by serial dilution.
Phage morphological analysis
PEG concentrated phages (108 pfu/mL) were spotted on a carbon coated grid, dried, stained with 2% phosphotungstic acid, dried and examined using Transmission Electron Microscope (Model Jeol/JEM 2100 2000X) operated at 200 kV to study morphology.
Optimal multiplicity of infection (MOI)
Optimal MOI was determined at with a ratio of 0.001, 0.01, 0.1, 1, 10 and 100. MOI giving maximum yield was considered optimal.12
Phage adsorption and one-step growth parameters
Phage adsorption and one step growth curve were determined. Briefly, exponentially grown Salmonella culture were mixed with each phage at an optimum MOI and incubated at 37 ̊C. Aliquots were sampled at 0, 10, 20, 30, 40, 50, 60 minutes time intervals after infection, filtered and rate of adsorption was determined according to Adams.13
In one step-growth experiment, briefly, mid-exponential growing cultures of Salmonella cells were harvested and resuspended in 0.25 volume of nutrient broth. Phages were added at a optimum multiplicity of infection and allowed to adsorb for 15 min at 37 ̊C, centrifuged, resuspended in 200mL broth. At 10-min intervals over 120 min, aliquots from each dilution were collected, filtered and phage titre was determined according to Adams.13,14 The latent period and burst size were calculated.15
Phage viability studies
Phage viability was assayed in buffers at different pH values ranging from 2 to 13; after incubation for 30 min at 37 ̊C. Viability at six temperatures (50, 60, 70, 80, 90,100 ̊C) for 15, 30, 60 and 180 seconds were studied by incubating 100μL of phage sample (106pfu/mL) at these temperature for required time. Influence of NaCl on viability was determined by incubation at the various molar concentrations of NaCl (0.1M, 0.25M, 0.5M, 0.75M, 1M, 2M, and 3M) for 30 min at 37 ̊C. In all the cases, phages that survived were diluted and counted immediately.15
Sugar inactivation
Inactivation by various sugars like dextrose, galactose, fructose, maltose, mannitol, mannose, lactose, rhamnose, ribose and xylose of phage was also studied.12
Effect of calcium on propagation
Effect of calcium ions on phage adsorption and propagation was determined.16 Phages were incubated with Salmonella culture in nutrient broth with and without CaCl2 (0, 10, 20 and 30 mM) and phage titre was determined according to Adams.13
Effect of factors on adsorption
Exponentially grown bacterial cultures were mixed with each phage at optimum MOI. To study the effect of temperature, the mixture was incubated at 0, 10, 20, 30, 35, 40, 45, 50 ̊C for 30 minutes. To study the effect of pH, the phages and the host bacterium were mixed in nutrient broth adjusted to different pH. The effect of NaCl was studied using nutrient broth with the different molar concentration of NaCl (0.1M, 0.25M, 0.5M, 0.75M and 1M). In all experiments, tubes were incubated for 30 min at room temperature, filtered and assayed using double agar overlay to determine the rate of adsorption.15
Survival of bacteriophage under nutrition depletion conditions
The capacity of bacteriophage to survive in nutrition deficient conditions was studied.17
In vitro lytic assay
An overnight culture of Salmonella strain was diluted to 1: 10 and was infected with phage at higher MOI of 10, incubated at 37°C for 6 h and OD600 was measured.
Host range analysis
The host range of the phages were examined using double agar overlay method against 20 strains of Salmonella from the culture collection of the Department of Biotechnology, Cochin University of Science and Technology.
Statistical analysis
All the experiments were conducted in triplicates and plotted with ±SD using Graph Pad Prism 6 (Graph Pad Software, Inc., San Diego, CA). In vitro lytic assay was statistically evaluated by one factor ANOVA using Graph Pad Prism 6
Bacterial isolation, identification and antibiotic sensitivity
Salmonella host designated as BT55CW isolated from the chicken waste was used as the host for the study. Antibiotic sensitivity test indicated that the strain was resistant to sulfamethoxazole/trimethoprim, nalidixic acid, tetracycline and cefuxomine, but was susceptible to ampicillin, azithromycin, cefixime, chloramphenicol, ciprofloxacin, and gentamicin. The MAR index was calculated to be 0.4.MAR index in an indicator that the strain is isolated from an environment where it is used frequently.
Bacteriophage isolation
Two lytic bacteriophages isolated using BT55CW as hosts were designated as ɸSEP2 (from chicken intestinal contents) and other ɸSEP3 (from slaughter house liquid waste) showed excellent bacterial lysis. Plaques formed by ɸSEP2 were smaller than that by ɸSEP3.
Morphological analysis
Phage morphology by TEM revealed ɸSEP2 and ɸSEP3 belonging to two different families of order Caudovirales. ɸSEP3 with a prolate head of diameter of 105+0.32nm and a contractile tail of 141± 35nm lengths was classified as Myoviridae, whereas ɸSEP2 with an icosahedral head having diameter of 94±16nm and extremely short tail belonged to family Podoviridae (Figure 1).
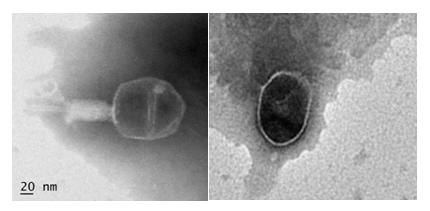
Figure 1 Transmission Electron Micrograph of phages stained with 2% phosphotungstic acid (A) ɸ SEP3 (20nm scale) (B) ɸSEP2 (50nm scale).
Morphology of ɸSEP3 was similar to the phage FGCSSa118 which had a mean head diameter of 107nm and tail length of 123 nm. The morphology of ɸSEP2 revealed greater than reported average head diameter (62.5nm) of phages belonging to family Podoviridae.19
Multiplicity of infection
The optimal MOI for both ɸ SEP3 and ɸ SEP2 was one phage per bacterium and was used for their large-scale production. Optimum MOI is important as low MOI can cause infection of only a very small proportion of cells; where as high MOI could cause ‘lysis from without’ where host cells adsorbing abundant phage particles are lysed rapidly even in the absence of phage replication.20
Phage adsorption and one-step growth curve
The replication dynamics of each phage-host system was characterized in vitro. For ɸSEP3 100% adsorption occurred after 50 minutes of incubation with the host, where as 90% ɸ SEP2 phage particles absorbed within 60 minutes (Figure 2A).
The one step growth curve analyzed latent period and burst size (Figure 2B). The latent period of ɸ SEP2 was 40 minutes with a burst size of 88 progeny viruses per bacterial cell. Many reported phages have a similar latent period.21 Though ɸSEP3 was characterized with a shorter latent period of 10 minutes, phage SE2 specific to Salmonella Enteritidis was reported with a similar latent period.22 The calculated burst size of ɸSEP3 was found to be 32 which is smaller than that of ɸSEP2. This could be by virtue of shorter latent period as larger burst sizes are associated with longer latent periods, but shorter generation times are associated with shorter latent periods.23
Phage viability studies
Resistance to high temperatures and pH are critical for bio-control applications. Influence of temperatures on phage viability is presented in Figure 3A & 3B. ɸ SEP2 was viable at 50 ̊C for 15 seconds, but at 60 ̊C for 15 seconds, PFU was reduced by >72% while viability was completely lost at the end of 1 minutes at 70 ̊C. In the case of ɸ SEP3, viability was completely lost at 70 ̊C for 3 minutes. ɸ SEP3 appears to be more tolerant to high temperature compared to ɸ SEP2.
Figure 3D represented the effect of pH on the viability of ɸSEP2 & ɸSEP3, where optimum pH for the survival of ɸ SEP2 and ɸ SEP3 was pH 7 and 8 respectively. ɸ SEP2 was stable over pH range 5-8 where as stability of ɸ SEP3 was over a broader pH range of 4-11. Both ɸ SEP2 and ɸSEP3 could tolerate upto 3M NaCl. The optimum for ɸ SEP2 survival was 0.5M NaCl (Figure 3C), but there was a reduction in viability at concentrations higher than 0.5M, whereas the optimum for ɸSEP3 for survival was only 0.25M NaCl.

Figure 3 Effect of temperature on viability of (A) ɸSEP2 (B) ɸSEP3 (C) Effect of NaCl on viability of ɸSEP2 and ɸSEP3 (D) Effect of pH on viability of ɸSEP2 and ɸSEP3.
Sugar inactivation
Carbohydrates present in the bacterial cell walls are used as receptors by phages. When they bind irreversibly to their isolated receptor, it resulted in inactivation.23 The sugars mimic disaccharide receptors and inhibit phage adsorption.24 The effect of ten different sugars on inactivation of ɸSEP3 & ɸSEP2 is depicted in Figure 4A & 4B. Arabinose caused 100% inactivation of ɸSEP3, while that by mannose, xylose and dextrose was 70%, 60% respectively and was 50% by rhamnose and maltose. Mannitol and lactose caused only 46% and 32% inactivation respectively. 20% inactivation by galactose was observed, but fructose had no effect. In the case of ɸSEP2 fructose, rhamnose, and dextrose caused severe inactivation, as much as 97%, 93% and 90 % respectively; 85% by galactose and lactose, while that by mannose was 83%. 75% inactivation by maltose and mannitol was noted, while that by xylose and arabinose was 82% and77% respectively. Both ɸSEP3 & ɸSEP2 were inhibited by sugars suggesting that the carbohydrates tested are essential components of phage receptor structures.
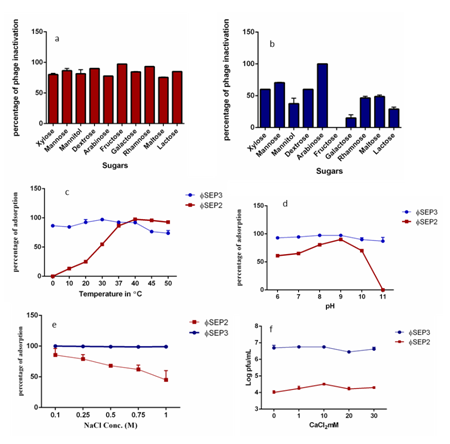
Figure 4 Sugar Inactivation of (A) ɸSEP2 (B) ɸSEP3 (C) Effect of temperature on adsorption of ɸSEP2 and ɸSEP3 (D) Effect of pH on adsorption of ɸSEP2 and ɸSEP3 (E) Effect of NaCl on adsorption of ɸSEP2 and ɸSEP3 (F) Effect of CaCl2 on propagation of ɸSEP2 and ɸSEP3.
Effect of calcium on propagation
Divalent metal ions, in particular, calcium ions improve the adsorption rate and control the penetration efficiency of phage DNA into the bacterial cells.25 The addition of CaCl2 caused an observable increase in titre of ɸ SEP2 and its propagation was optimum at 10mM CaCl2. However CaCl2 did not affect the propagation of ɸSEP3, nevertheless a drastic dip in the viral count was observed at 10mM (Figure 4F).
Adsorption of phages depends not only on the receptor, but also on different environmental factors.26 It was observed that phage adsorption varied at different temperatures, maximising at 30 ̊C-40 ̊C (Figure 4C). Maximum adsorption of ɸ SEP2 nearing 100% occurred at 40 ̊C. 14% adsorption of ɸ SEP2 occurred at 10 ̊C which accelerated to 90% at 37 ̊C. More than 90% adsorption was still till 50 ̊C. ɸSEP3, considerable adsorption occurred at all temperature studied, with maximizing at 30 ̊C (97%). Though phage activity was better at the higher temperatures, this may not hamper application at low temperatures as both phages showed adsorbance at lower temperatures, with > 80% adsorption occurred at 0 ̊C for ɸ SEP3. Ability to adsorb at low temperature makes ɸSEP3 a candidate biocontrol agent for cold storage of poultry products. Phage adsorption is intensely influenced by pH of the medium. Maximum adsorption of ɸ SEP2 was at pH 9 (90%) at 30 minutes of incubation with host. From pH 6-10 adsorption occurred at a range of 60%-90 % (Figure 4D). For ɸ SEP3, adsorption was > 90% in pH ranges studied. 97% percentage adsorption at pH 8 was considered optimum. In varying concentrations of NaCl, ɸ SEP2 adsorbed maximally at 0.25M NaCl, while ɸ SEP3 showed > 90% adsorption at all concentrations tested (Figure 4E & 4F).
ɸ SEP2 and ɸ SEP3 lysed 60% of all Salmonella strains tested (data not shown). Host range results were identical for ɸ SEP2 and ɸ SEP3 suggesting that they recognise identical or very similar receptors in their host.
Survival under nutrient-deprived conditions
ɸ SEP2 and ɸ SEP3 survived nutrition depleted conditions of host and this ability of bacteriophage to propagate is crucial. In our opinion, evaluating this ability should be a part of the routine characterization of bacteriophage before using it as a bio-control agent. Laboratory conditions do not correspond to natural settings. Competition for nutrients will be very high in a natural environment. Studies have shown that bacteriophage lytic development can be inhibited proficiently by carbon source limitation in bacterial cultures.27 Development of the phages depend on intracellular resources of their hosts, which in turn depend on the physiological state of their hosts.28-30 ɸSEP2 and ɸSEP3 survived at all conditions studied with a maximum of one log reduction when the host was in starved state (Figure 5A) (Figure 5B). When compared to already reported phages, (17) ɸ SEP2 and ɸ SEP3 survival was exceedingly well making them potential candidates for bio control applications.
In vitro lytic assay
Both phages significantly reduced the Salmonella at the tested MOI proving their efficiency to reduce growth of Salmonella (Figure 5C).
The study was carried out to isolate bacteriophages to be part of cocktails for targeting Salmonella. Characterization of phages is important for its use as suitable bio-control agent. It was interesting that ɸ SEP3 was stable to all the factors studied and could effectively adsorb in all conditions when compared to ɸ SEP2 and many of the reported phages. One reason could be the source of the phage, as ɸ SEP3 was isolated from an environment where there is greater variation in factors/conditions, whereas ɸ SEP2 was isolated from chicken intestine where conditions are stagnant/stable. Resistance to environmental factors is critical as well as its lytic spectrum. Effective treatment requires phages of high lytic activity. Both phages could equally reduce the growth of Salmonella in the significant level. In brief, we can say ɸ SEP3 is a better candidate but conclude that multiple factors need be analyzed before using as bio control agent.
The work was supported by Kerala State Council for Science, Technology and Environment (KSCSTE) through the project F.No.009/SRSHS/2012/CSTE. The authors would like to acknowledge Department of `Biotechnology, Cochin University of Science and Technology (CUSAT) for providing all the facilities for research. Author is grateful to Laxmi M and Jeena A for their guidance. STIC, CUSAT is acknowledged for TEM analysis.
The author declares no conflict of interest.

©2017 Sritha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.