Journal of
eISSN: 2469 - 2786


Review Article Volume 7 Issue 6
1Deparment of Biology, Akdeniz University, Turkey
2Deparment of Food Processıng, Osmaniye Korkut Ata University, Turkey
Correspondence: Hasan AKGÜL, Deparment of Biology, Faculty of Science, Akdeniz University, Turkey
Received: August 07, 2019 | Published: November 27, 2019
Citation: SEVİNDİK M, AKGÜL H. Fruiting bodies structures of myxomycetes. J Bacteriol Mycol Open Access. 2019;7(6):144-148 DOI: 10.15406/jbmoa.2019.07.00260
Myxomycetes (plasmodial slime molds) are very important living groups in ecosystem and cosmopolitan species. In this study, morphological structures (Hypothallus, Stalk, Columella, Pseudocolumella, Capillitium, Pseudocapillitium, Peridium, Cortex, Calyculus and Spores) of myxomycetes are given. The structures have supported by the examples shown.
Keywords: myxomycetes, plasmodial, mushrooms, yeasts, fungi hyphae, blue-green bacteria, hypothallus, fruiting bodies
Myxomycetes, known as true slime mushrooms or plasmodial slime mushrooms, are multi-nucleated, non-cell walled creatures capable of producing one or more spores. They are fungi-like organisms that are very common in terrestrial ecosystems. They are surrounded by a transparent adhesive scabbard and thin plasma membrane. Plasmodium in the form of a multi-nucleus and acellular protoplasm stack represents the vegetative phase. Plasmodium formation and subsequent haploid chromosome number spores include one or more fructification. Plasmodium is formed by germination of spores. In the sporulation phase, these organisms form a distinctly formed spore mass within the membranous spore sac secreted by the protoplasm. The spore sacs also contain a system of non-cellular, mostly free or reticulated yarns capillitium or pseudocapillitium. Some groups have systematically important characteristic lime deposits inside, outside, or both places in the spore sac.1,2 Some species are very common, while others live in certain habitats. Myxomycetes are sensitive to light, moisture and temperature as well as the property of the substrate on which they develop. Mycetozoa members are abundant in cool, damp and shady environments such as decaying stumps, branches, live or dead tree bark, rotten fruit or fruit residues, dead leaves and leaf debris. In addition, some organic materials were collected as exceptional from herbivorous animal feces and rocks. They live in such environments by feeding with other microorganisms (such as bacteria, yeasts, fungi hyphae, blue-green bacteria, and green algae). By collecting materials that may contain spores and plasmodium from the natural environment, sporophore development is provided by the Humidity Chamber Technique in the laboratory.3‒7 The fruiting bodies of myxomycetes can occur anywhere under optimum conditions (proper humidity, heat and sufficient rotting organic matter). Many myxomycetes species have small fruit stems. It is only a few millimeters in diameter and requires a 20 × lens for fixation.8 Myxomycetes have four fruiting body forms or types, sporangium, plasmodiocarp, aethalium and pseudoaethalium. The most common fruiting body forms are a stalked sporangium and heterothallic myxomycetes sporangium contains mainly 7 parts.
Hypothallus
The Hypothallus is a thin layer deposited by plasmodium at the time of fruiting on the surface of the substratum. The hypothallus connects the stalk to the substrate. The hypothallus may be composed of CaCO3 , also membranous and transparent. It is relatively thick from the membrane. It can be of different colors, from delicate, hard and transparent to bright. The hypothallus may surround the base of a single fruiting body. And also may form a continuous layer that joins the bases of a plurality of fruiting bodies. The hypothallus may not be apparent at all in some myxomycetes7,9 (Figure 1 & 2).

Figure 1 Didymium squamulosum (Alb. & Schwein.).8

Figure 2 Arcyria denudata (L.).8
Stalk
In certain fruiting bodies, sporangium is elevated above the substrate by a stalk (sometimes also called a stipe). The primary function of the stalk is to support and elevate the spores above the substratum. Ayrıca protecting the sporangium from excessive moisture and aiding in the dissemination of the dry, powdery spore mass. Stalks display considerable variation in length, thickness, color, and texture. Moreover, they may be hollow or filled with one of several types of material (for example, round, sporelike structures or granular debris), translucent or opaque, and covered with lime or limeless. The stalk may be useful in identifying myxomycetes such as in the Order Physarales where the stalk contains CaCO3 and in the Order Stemonitales where the stalk is either hollow or filled with fibrous strands, or filled with sporelike structures in Arcyria (Figure 3 & 4).7,9,10

Figure 3 Badhamia dubia Nann.-Bremek.10

Figure 4 Comatricha tenerrima.10
Columella
In some fruiting bodies, the columella is often an extension of the stalk within the peridium. The columella may be various sizes as dome-shaped, spherical, or elongated central sterile structure within the sporangium. In stalked fruiting bodies, the columella appears to represent an extension of the stalk that simply continues upward into the spore mass, although it may or may not resemble the stalk in structure, shape, texture, and color. In sessile fruiting bodies, the columella ranges from little more than a thickening of the inner side of the peridium where it contacts the substrate to a conspicuous dome-shaped structure. Columella may serve as a supporting structure for the capillitium that may be attached in part or throughout its length. Columella may be a short protrusion or extend to the top of the sporangium. Columella is an important part of the definition when it occurs. Used in keys to identify different types11,9 This structure is absent in species of the Liceales and Trichiales12 (Figure 5 & 6).

Figure 5 Comatricha nigra (Pers.).12
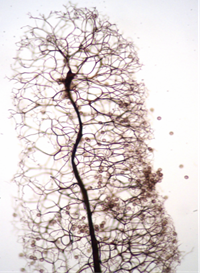
Figure 6 Stemonitis herbatica Peck.12
Pseudocolumella
A pseudocolumella (literally, a false columella) is a structure that resembles a columella. Pseudocolumella does not attach to the stalk. Pseudocolumella is a lime mass freely suspended in the center of the sporangium, resembling a true columella. The lime mass may consist of a single discrete body or may be represented by a loose aggregation of smaller units.7,9 The pseudocolumella is found only in the order Physarales, The example is Craterium leucocephalum Ellis (Figure 7).

Figure 7 Craterium leucocephalum.13
Capillitium
The capillitium (plural: capillitia) is a system of threadlike elements found within the spore mass of the fruiting body in many different species of myxomycetes. Portions of the capillitium may be solid or hollow, free or interconnected, smooth or sculptured. A capillitium containing lime (a badhamioid capillitium) is present only in some members of the Physarales and may be entirely limy or consist of a system of limeless, hyaline tubules connecting conspicuous deposits of lime usually referred to as lime nodes(a physaroid capillitium). These threads form from a system of preformed vacuoles that coalesce, giving rise to solid or hollow threads often with debris and of uniform diameter usually less than 6.0μm.7,9. Threads in Arcryia species are more or less elastic and expand at maturity13 (Figure 8 & 9).
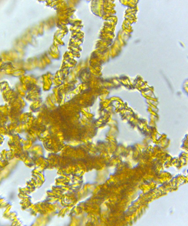
Figure 8 Hemitrichia montana (Morgan) T. Macbr. 12
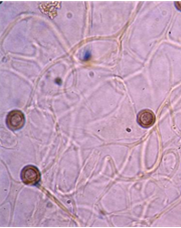
Figure 9 Willkommlangea reticulata (Alb. & Schwein.)14
Pseudocapillitium
Pseudocapillitia occur in pseudoaethalia and aethalia those produced by certain members of the Liceales. Unlike the capillitium, where the individual elements are more or less uniform with respect to both overall structure and diameter, the elements making up the pseudocapillitium are distinctly irregular and vary in width or diameter. Pseudocapillitial elements are highly variable in size and shape, and may appear as bristles, threads or perforated plates. Occurring as thread-like, membrane-like, or perforated structures intermingled with the spore mass in Reticularia species. Appears as a system of irregular tubes in aethalia of Lycogala species, such as L. epidendrum, where margins are wrinkled and much wider than 6μm in diameter (Figure 10 &11).7,9,14
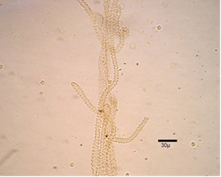
Figure 10 Lycogala epidendrum (J.C. Buxb. ex L.) Fr.15
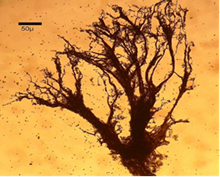
Figure 11 Amaurochaete atra (Alb. & Schwein.) Rostaf.14
Peridium
Peridium is that encloses the spores in the endosporous myxomycetes. It varies in structure from delicate, thin, and membranous to relatively tough and thick. In some myxomycetes, the peridium is not evident in mature fruiting bodies. Some peridia are brilliantly colored in hues of orange, yellow, violet, and red. Also others are iridescent or infused with calcium carbonate (CaCO3). When maturity, the peridium may be persistent as in Perichaena. Also it may be persistent either single-layered as in P. microspora, double as in P. depressa, or triple as in Physarum bogoriense.7,9 Peridium may be membranous and evanescent as in species of Macbrideola or Stemonitis (Figure 12 & 13).15

Figure 12 Prototrichia metallica (Berk.) Massee16

Figure 13 Diderma radiatum (Rostaf.) Morgan10
Cortex
The rather thick covering that surrounds the spore mass in an aethalium is usually referred to as a Cortex. Cortex is thickened calcareous outer covering of the aethalium in the genus Fuligo. This structure appears in all Fuligo species descriptions instead of the term peridium. Because it generally functions in the same way, protecting the black spore mass inside. Fuligo septica (L.) F.H. Wigg is a conspicuous example because of its large size and welldeveloped cortex of calcareous granules. Mucilago crustacea P. Micheli ex F.H. Wigg. also has a thickened cortex. But in this case, it is composed of crystalline not granular calcium carbonate. It is a look-alike species sometimes misidentified as F. septica since both are common species found in similar habitats (Figure 14).
Calyculus
A cup, referring to the persistent peridium forming a cup at the base of a sporangium. Most species in the genera Arcyria and Cribraria have a calyculus. Depth and surface markings on the cup can be used for species identification (Figure 15 & 16).16

Figure 15 Arcyria minuta Buchet17
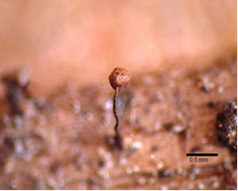
Figure 16 Cribraria piriformis Schrad8
Spores
Spores are the reproductive units, microscopic walled haploid, formed inside the fruiting body. The spores of virtually all myxomycetes are spherical and those of most species have a diameter that falls between 5 and 15 micrometers. In species identification, the color, shape, size and ornamentation of spores can be quite useful.9,10 If spore size varies greatly for spores from the same fruiting body, it almost invariably indicates that the fruiting body did not develop normally. Spore color in myxomycetes varies from hyaline to almost black. Also the only colors not found are blue and true green. Spores usually are spherical in shape. Also it is free as single units or aggregated into loose or tight clusters. The various types of ornamentation that can be present on the spores of a particular species include asperulate (roughened or densely but very finely warted), verrucose (with scattered but relatively prominent blunt warts), echinate (with scattered but relatively prominent sharp-pointed spines), and reticulate (covered by a network of ridges). Spores are either dark, as is the case for most members of the Physarales and Stemonitales or pale to brightly colored, which is the condition that exists for all other myxomycetes. Brightly colored spores may be red, yellow, orange, white, pale gray, pink, light or rusty brown. In some species of Badhamia and Dianema corticatum, the spores appear clustered into spore balls (Figure 17 & 18).9,10,17,18
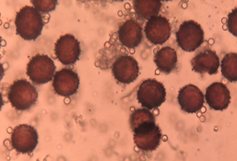
Figure 17 Physarum bitectum G. Lister18

Figure 18 Hemitrichia montana (Morgan) T. Macbr.12
Hypothallus, Stalk, Columella, Pseudocolumella, Capillitium, Pseudocapillitium, Peridium, Cortex, Calyculus and Spores structures of mycomycetes have been explained with examples. In this study fruiting bodies structures of myxomycetes have presented.
None.
None.
The author declares that there is no conflicts of interest.

©2019 SEVİNDİK, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.