Journal of
eISSN: 2469 - 2786


Review Article Volume 12 Issue 1
Narayan Consultancy on Veterinary Public Health and Microbiology, India
Correspondence: Mahendra Pal, Narayan Consultancy on Veterinary Public Health and Microbiology, B-103, Sapphire Lifestyle, Gujarat, India
Received: February 26, 2024 | Published: March 21, 2024
Citation: Pal M. Cryptococcus neoformans and cryptococcosis: a contribution made by Prof. Dr. Mahendra Pal. J Bacteriol Mycol Open Access. 2024;12(1):25-29. DOI: 10.15406/jbmoa.2024.12.00368
Mycotic infections are reported to occur in both sexes, all age groups, in all seasons, and in urban and rural areas of the world. The people with weakened immune systems are more likely to get an infection due to fungi, which are widely prevalent in our environment. Cryptococcosis primarily caused by Cryptococcus neoformans is a life-threatening enigmatic mycosis of global importance. It is estimated that 1000000 cases of cryptococcal meningitis complicating HIV/AIDS are reported every year worldwide. Disease has been encountered in humans as well as in a variety of animals including cats and dogs. The source of infection is exogenous, as the fungus occurs as a saprophyte in nature. Currently, the pigeon excreta serve as the chief saprobic reservoir of C. neoformans. Human as well as animals may acquire the infection from the environment where the fungus grows luxuriantly. The respiratory tract is recognized as the principal mode of entry of the organism. The pathogen can also enter through the fungal contaminated objects via traumatized skin. Clinical spectrum of disease is varied, as manifested with several forms, such as pulmonary, meningeal, visceral, osseous, and cutaneous. The laboratory help is imperative to confirm an unequivocal diagnosis of cryptococcosis. The pathogen can be easily isolated from the clinical specimens of the patients on Pal sunflower seed medium. Modified Pal sunflower seed can be employed to undertake the sexual compatibility studies of Cr. neoformans. Several drugs including amphotericin B, fluconazole, and itraconazole have been recommended for the treatment of disease. The immediate attention to the skin injury and use of face mask when dealing with avian droppings can help in the prevention of infection.
Keywords: Cryptococcus neoformans, cryptococcosis, AIDS, meningitis, pal sunflower seed medium, public health
Fungal diseases are an important cause of morbidity as well as mortality in humans and animals including birds.1,2 Everybody is at risk of getting the fungal infection mainly from the environment as fungi occur as saprobe in nature.1 It is estimated that mycotic infections affect more than a billion and kill over 1.5 million people worldwide.3 However, the fungal infections are still considered a neglected problems by the public health authorities.3 Several factors, such as HIV/ AIDS, diabetes, asthma, cancer, organ transplantation, cystic fibrosis, prolonged use of corticosteroid, antibiotic and neoplastic drugs, catheterization, and tuberculosis predispose the patients to fungal infections.1–3 Early correct diagnosis and prompt antifungal treatment is highly imperative to prevent the suffering of the patients due to mycotic diseases.1
Cryptococcus neoformans the chief cause of cryptococcosis, is a basidiomycetous yeast that occurs as a saprobe in a variety of environmental materials, such as avian droppings, wood, air, fruits and vegetables, bat guano etc.4–11
Cryptococcal infection has been described in humans as well as in many species of animals including cats, dogs, cattle, buffaloes, goats, sheep, monkey etc.1,12–17 The pathogen can infect many organs of the body, such as the skin, lung, bone, brain, eye, adrenal gland, lymph node, mammary gland and others.18 The infection has been described in immunocompetent as well as immunocompromised subjects.18
Mycological, immunological, and molecular techniques are used to establish the diagnosis of disease (1). Direct demonstration and isolation of Cr. neoformans from the clinical specimens is considered as the gold standard of diagnosis of disease.19
Pal sunflower seed medium (Pal medium), which was developed in 1980 has been employed to make an unequivocal diagnosis of cryptococcosis in humans and animals.20 The light to dark brown -coloured colonies of Cr. neoformans on Pal medium helps in the early and correct diagnosis of cryptococcosis. In addition, Pal medium proved very useful for conducting epidemiological studies on cryptococcosis.1 It is pertinent to mention that actidione should not be added in the medium as it inhibits the growth of Cr. Neoformans.18 Cryptococcus neoformans is an encapsulated, basidiomycete fungus pathogen that produces the enzyme phenoloxidase, which is necessary in melanin synthesis. Melanin production by Cr. neoformans on Pal medium is an important phenotypic character that helps in an early identification of this zoopathogenic basidiomycetes yeast from clinical as well as environmental sources.21 Furthermore, it has been studied that both species of Cryptococcus i.e. neoformans and gattii has produced melanin on Pal medium as evidenced by dark brown pigment.8
The microscopic morphology of isolates of Cr. neoformans can be studied in PHOL (Pal, Hasegawa, Ono, Lee) stain (Formalin (4%) 5.0 ml, Glycerol 3.0 ml, and Methylene blue (3%) 0.3 ml,22 or Narayan stain ( Dimethylsulphoxide (DMSO) 6.0 ml, Methylene blue 0.5 ml and Glycerine 4.0 ml).23
The sexual state of Cr. neoformans was described for the first time by Kwon-Chung.24 The yeast occurs in two mating types namely “a” and “alpha”. Several media like malt extract agar, sucrose yeast extract biotin agar, hay infusion agar, V-8 juice agar are employed to undertake in vitro genetic crossing in Cr. neoformans. In this context, Pal8 used modified Pal sunflower seed medium (modified Pal medium) for studying sexual reproduction.8 When two mating types “ a” and “ alpha” are crossed on modified Pal medium, the perfect state of Cr. neoformans is formed. The isolates of Cr. neoformans from different countries including Belgium, Japan, India, and Nepal belonged to “alpha” mating type of Filobasidiella neoformans var. neoformans.8,25–31
Several antifungal drugs, such as amphotericin B, flucytosine, ketoconazole, fluconazole, itraconazole, and liposomal amphotericin B are used for the treatment of cryptococcosis.1,17 Currently, there seems to be no effective chemotherapeutic agent commercially available to treat the cases of cryptococcal mastitis in dairy animals.32
Globally, one million cases of cryptococcal meningitis occur annually in HIV/AIDS patients resulting in about 625,000 deaths.33 It is mentioned that over 80% of the patients suffering from the pulmonary cryptococcosis gave a history of exposure to environment.34 Furthermore, over 90% of patients with pulmonary cryptococcosis were misdiagnosed and among them, 50% received the treatment for pulmonary tuberculosis.34 The present communication delineates the last five decades contribution of Prof. Dr. Mahendra Pal in Cryptocococcus neoformans and cryptococcosis.
Cryptococcus neoformans
The ecological association of C. neoformans with avian excreta was established for the first time by Emmons who isolated the fungus from the pigeon (Columbia livia) droppings in USA.35 Later, several investigators from many countries of the world including India confirmed that Cr. neoformans occurs in the faecal matter of pigeons.4,5,11,31,36–38 In this context, Pal and Baxter39 established the occurrence of Cr. neoformans for the first time in the environment of New Zealand. Likewise, Pal in 199731 was the first to demonstrate the prevalence of Cr. neoformans in Nepal by isolating the fungus from the pigeon droppings on Pal medium. The in vitro genetic crossing studies attempted on modified Pal sunflower seed medium (Modified Pal medium) for the first time8 indicated that all the strains of Cr. neoformans originated from Nepal belonged to “alpha” mating types. The pathogen was also recovered from the excreta of pigeons from Djibouti (Africa) for the first time in 2015 by Pal.38 In addition, Cryptococcus neoformans has also been isolated from the parrot droppings, bat guano, soil, fruits and vegetables, wooden nest boxes and perches, and other avian excreta, such as parrots, munia birds, lorikeet, and budgerigar.1,4–8,10,11,25,40 The persistence recovery of Cr. neoformans from the environment of a zoo aviary in Antwerp, Belgium was a noteworthy observation.8 The pathogen was isolated from the empty wooden canary cages that were lying unused for the last eight months in the basement of a building in Antwerp, Belgium.29 However, the droppings of pigeon serve as the principal saprobic reservoir of C. neoformans. It is important to cite that Cr. neoformans can survive for two decades in old and dry pigeon excreta that was protected from direct ultraviolet rays of sun.19 Direct exposure of sunlight is detrimental to the survival of Cr. neoformans in the pigeon droppings.1 Recently, Pal21 described the isolation of Cr. neoformans from the dust of air conditioners on Pal medium for the first time in Bharuch, Gujarat, India.
Cryptococcus neoformans can be easily isolated from clinical as well as environmental materials on Pal medium that contained 45 g pulverized sunflower seed, 10 g agar, 100 mg chloramphenicol and 1000 ml distilled water.20 The identification of yeast on Pal medium can be easily made by observing light brown to dark brown pigmented colonies of C. neoformans (Figure 1). The brown colour effect is due to the presence of melanin. Both species of Cryptococcus i.e. neoformans and gattii has produced melanin on Pal medium as revealed by dark brown pigment.8

Figure 1 Several brown-pigmented colonies of Cryptococcus neoformans on Pal medium isolated from the clinical specimen of a 79-year-old woman who had lung cancer as an underlying disease.
The clinical and environmental isolates of Cr. neoformans when examined in PHOL22 or Narayan stain23 under light microscope revealed many spherical to few oval shaped thinly encapsulated yeast cells, with and without budding.1
The sexual compatibility study is important from epidemiological point of view. Sexual reproduction of Cr. neoformans isolates from clinical and environmental sources was conducted on modified Pal medium to know the mating type (Figure 2). It was interesting to know that all the clinical and environmental strains of C. neoformans originated from several countries including Belgium, Japan, Nepal, and India belonged to mating type “alpha”.8,25,27–31 These observations reinforced that modified Pal medium serves an excellent tool to study the prevalence of mating types of Cr. neoformans.
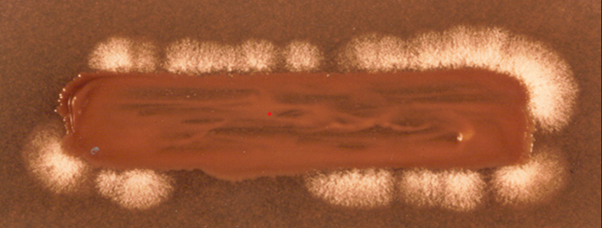
Figure 2 Sexual reproduction of an environmental isolate of Cryptococcus neoformans with known tester strain of Filobasidella neoformans var. neoformans “ a” mating type on modified Pal medium showing white mycelial growth over the margin of culture streak indicating that the isolate belonged to “ alpha” mating type of Filobasidella neoformans var. neoformans.
A number of laboratory animals, such as mice, rat, Guinea-pig, rabbit, hamster etc. are employed by the scientists to conduct experimental research.1 Among these, laboratory animals, mice are most commonly used for pathogenicity test of the isolates of Cr. neoformans recovered from environment.41 Experimental studies have confirmed that the environmental strains of Cr. neoformans were found pathogenic to the laboratory mice when inoculated intracerebrally.41 The yeast was reisolated from the brain tissues of the autopsied mice and also demonstrated in the impression smears of the brain by Mayers mucicarmine and periodic acid-Schiff (PAS) staining technique (Figure 3).
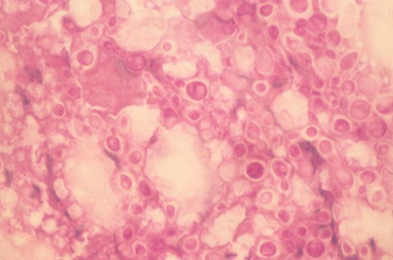
Figure 3 Impression smear prepared from the brain of a mouse scarified after ten days of inoculation of Cryptococcus neoformans isolate from an AIDS patient, showing typical enlarged capsules of Cryptococcus neoformans. Periodic acid-Schiff × 250.
Cryptococcosis
Cryptococcosis is an emerging life-threatening infectious enigmatic fungal saprozoonosis of global importance.19 It is an acute, subacute and chronic mycosis that is caused by the genus Cryptococcus, consisting of 37 species of which C. neoformans and C. gattii are implicated in most cases of cryptococcosis.14 Cryptococcosis occurs in sporadic and epidemic form resulting in high morbidity and mortality in the susceptible hosts. The respiratory tract is documented as the prime mode of entry of the yeast, and the source of infection is exogenous. It is thought that humans and animals may acquire cryptococcal infection from saprobic reservoirs where the fungus grows luxuriantly. The ubiquitous occurrence of the fungus in nature can become a significant source of cryptococcal infections to humans and animals. The disease can occur in several forms, such as pulmonary, meningeal, visceral, cutaneous and osseous.18 Numerous antifungal drugs, such as amphotericin B, flucytosine, ketoconazole, fluconazole, itraconazole, and liposomal amphotericin B are available for the treatment of cryptococcal infections.1,17,18
The first record of cutaneous cryptococcosis in India was published by Pal and Mehrotra.42 Cryptococcus neoformans was isolated from the cutaneous lesions of a pet cat on Pal medium. Generalized cutaneous mycosis was described in another cat that was unresponsive to amphotericin B therapy (Figure 4). Later, the cat was euthanized after seeking the written consent of the owner.
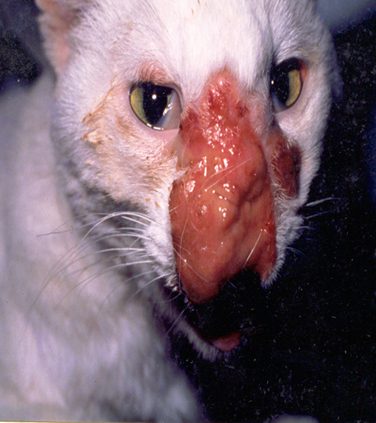
Figure 4 Cutaneous cryptococcosis in a 3-year-old female domestic cat with presence of mucoid, granulomatous mass on the nose. The yeast was isolated on Pal medium from the biopsy of the skin lesion.
Cryptococcal mastitis is a serious fungal disease of dairy animals, such as cow, buffalo, goat, and sheep.1,14–16 The mastitis has been reported to occur in sporadic as well as in epidemic form. The etiologic role of Cr. neoformans in mastitis in goat and buffalo was elucidated for the first time in 1975 and 1980, respectively.43,44 The affected animals show the swelling and induration of mammary gland, and yeast was recovered from the mastitic milk on Pal medium. The treatment of mycotic mastitis is challenging as presently available drugs failed to show good response.32
A study on cryptococcal mastitis was conducted on 79 dairy animals (41 buffaloes, 23 cows, 8 sheep and 7 goats.12 Cryptococcus neoformans was isolated from the mastitic milk of 2 buffaloes and 1 cow.
Pal and Dave18 recorded a case of mastitis in a dairy goat by recovering Cr. neoformans from the milk of the affected quarter on Pal medium (Figure 5).
Pal and co-workers13 recorded the pulmonary cryptococcosis in 5-year-old rhesus monkey for the first time in India. The affected monkey was treated for bacterial pneumonia but there was no response and the animal died. The pathogen was isolated from the lungs of the autopsied monkey on Pal medium. Histopathological examination of lung tissue of pet monkey revealed numerous encapsulated cells of Cr. neoformans in PAS stain.
Cryptococcal meningitis was confirmed for the first time from India in 2 dogs by isolation of Cr. neoformans from brain tissue of the necropsied dog. The histopathological examination the brain tissue showed a large number of yeast cells with and without budding in Mucicarmine stain. The epidemiological investigation revealed the source of infection in one of dogs by recovering the fungus from the pigeon excreta where the animal was kept. It is suggested cryptococcosis should be considered in the differential diagnosis of canine.14
Fatal meningitis in a cat due to Cr. neoformans var. neoformans was diagnosed by Pal.16 The pathogen was cultured from the brain tissue of the affected animal. This constitutes the first report of meningitis in a cat from India.
Pulmonary mycosis due to Cr. neoframns was diagnosed in a pigeon handler who was continuously exposed to the infectious cells of yeast.
The pathogen was isolated from the sputum of the pigeon handler on Pal medium. The detailed typing of the isolate on modified Pal medium indicated that it belonged to “alpha” mating type of Filobasidiella neoformans var. neoformans.28
Disseminated cryptococcosis was diagnosed in a 4-year-old German Shepherd dog with a history of respiratory disorder. Despite ketoconazole therapy, the animal died. Encapsulated yeast cells of Cr. neoformans was demonstrated in the impression smear of cutaneous granuloma by Periodic acid Schiff (PAS) staining technique. The pathogen was isolated from the urine sediment, skin biopsy, and lymph node aspirate on Pal medium.30
Cutaneous mycosis was encountered in a 34- year- old male parrot enthusiast who received a minor traumatic injury on the hand during the act of cleaning the cage. The patient was immunocompetent and was continuously exposed to the excreta of a caged parrot. The biopsy from the cutaneous lesion of a parrot keeper was examined mycologically. Microscopic examination of skin biopsy in India ink mount showed many thickly encapsulated yeast cells of Cr. neoformans. Many dark brown pigmented colonies of Cr. neoformans were isolated from biopsied tissue on Pal medium. The microscopic morphology of the fungal isolates was done in Narayan stain. There was no growth of Cr. neoformans from urine and blood of parrot keeper on Pal sunflower seed medium. Epidemiological findings established that the patient contracted primary cutaneous cryptococcosis from his immediate environment as indicated by the presence of Cr. neoformans in the excreta, and wooden cage of the caged bird. The patient was treated with fluconazole, an antifungal antibiotic (200 mg once daily orally) for 14 weeks.17 The drug was well tolerated and no side effects were noticed.
Cryptococcosis is a highly infectious life-threatening mycosis of humans as well as animals. The disease has been reported from the developing and developed nations of the world. The causative agent Cr. neoformans can survive in the pigeon dropping for a long period of time. Humans and animals may acquire cryptococcal infections from saprobic reservoirs where the fungus grows luxuriously. Maximum cases are recorded in the immunocompromised patients. The prompt diagnosis and immediate therapy is recommended to save the life of patient. The immunocompromised individuals are advised not to visit the sites that are contaminated with avian droppings. Pal medium being very cheap selective medium should be routinely employed in the microbiology and public health laboratories for making a confirmatory diagnosis of cryptococcosis besides conducting epidemiological investigation and sexual mating. Further research on the ecology, epidemiology, chemotherapy, and vaccinology will be rewarding.
None.
The authors declare that there are no conflicts of interest.

©2024 Pal. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.