Journal of
eISSN: 2469 - 2786


Case Report Volume 7 Issue 5
1Federal University of Goiás, Brazil
2Federal University of Recôncavo da Bahia, Brazil
3Federal University of Brasília, Brazil
Correspondence: Clever Gomes, Associate Professor, Institute of Biological Sciences, Federal University of Goiás. Campus Samambaia Avenida Esperança s/n. CEP 74690-900 Goiânia, Goiás, Brasi, Tel 55 62 3521-1115 & 55 62 99213-1894
Received: October 18, 2019 | Published: October 25, 2019
Citation: Gomes C, Santos EJ, Silveira-Lacerda E, et al. Blood transfusion and diagnostic accuracy of chagas disease. J Bacteriol Mycol Open Accessm. 2019;7(5):112-114. DOI: 10.15406/jbmoa.2019.07.00255
Introduction: Disclaim of the Chagas disease case in Blood Center is based on the immunofluorescence and enzyme-linked immunosorbent antibody assays.
Methods: The antibody assays were conducted in parallel with the nuclear DNA-PCR test in blood samples of 145 families’ members.
Result: The specific antibody tests were positive in 26.9% (39/145) subjects. Contrastingly, the Trypanosoma cruzi nuclear DNA-PCR was positive in 72.4% (105/145) of those blood samples.
Conclusion: The immune tolerance herein explains the reported discrepancies of results between the specific antibody and the accurate DNA analysis. The warranty of safe blood transfusion is achieved by the PCR technology.
Keywords: Chagas Disease; immune tolerance; nuclear DNA-PCR; diagnosis accuracy; blood transfusion.
Chagas disease used to be limited to rural regions of the American Continent infested with triatomine (Reduviidae:Triatomine) bug transmitter of Trypanosoma cruzi infection.1 The epidemiological profile changed as a consequence of social and economic a cause that underlies the human exodus mostly of the South and Central America to the Northern Hemisphere. Now present in five Continents the disease poses a growing burden to the public health systems.2-7 The infection can be transmitted congenitally by a Chagas’ mother to the offspring, by blood transfusion, by organ transplantation, by the ingestion of contaminated food, and by accident in the research laboratory and health facilities. In addition, the T. cruzi can be transmitted sexually to naive females and males.8-10
Acute Chagas disease with fever and malaise usually subsides on average of three months. The chronic infection can continue for life without clinical manifestation. However, the pathology manifests in one-third of those with Chagas heart disease (Figure 1), mega colon and mega esophagus (Figure 2).10 The acute infection is diagnosed by parasitological demonstration and by specific IgM antibody 15 days after parasite acquisition10. The chronic T. cruzi infection specific IgG antibody is showed by indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA). The health facilities rely on trade mark kits of these assays to disclaim blood donors, and an automated chemiluminescent kit showing 97.6% specificity can be used for the diagnosis of Chagas disease in health facilities.8
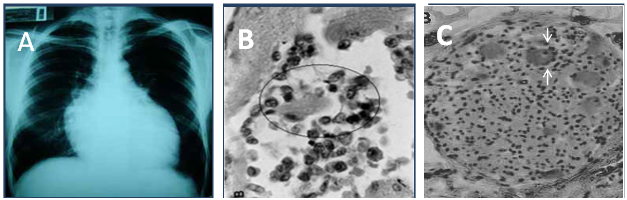
Figure 1 The heart pathology in Chagas disease. A) Cardiomegaly shown by chest X-Rays. B) Rejection and lysis of parasite-free heart cells (circle) by autoimmune lymphocytes. C) Rejection of parasympathetic neurons (arrows) by lymphocytes. Photographs from doctor Teixeira’s file.
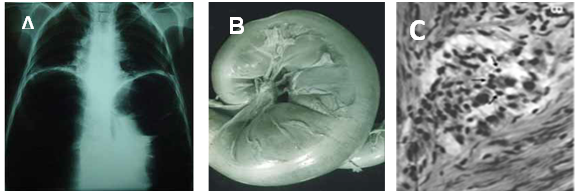
Figure 2 The digestive tube pathology in Chagas disease. A) Mega esophagus shown by barium sulphate contrast and X-Rays. B) Gross dilation and thickening of the colon wall. C) Rejection of parasympathetic neurons (arrows) by lymphocytes. Photographs from doctor Teixeira’s file.
However, the varying sensitivity and specificity of methods to diagnose the Trypanosoma cruzi infection has raised concerns regarding the safety and accuracy of blood donor disclaimers. In this regard, a PCR technology that discloses 10 femtograms of T. cruzi DNA, which is 1/24 of the total diploid parasite DNA, is recommended11. The specificity of nuclear DNA-PCR, Southern blotting with radio labeled specific 188-nucleotides telomere sequence, cloning and sequencing of amplicons (nDNA-PCR) was noted in 21 cases of acute Chagas disease (Figure 3) with parasitological demonstration.12,13 The reliability of the methods was assessed in double-blind control experiments that included de-identified DNA samples from chronic Chagas disease cases with the parasitological demonstration. The point of care nDNA-PCR achieved the diagnosis of Chagas disease in blood samples of 109 subjects of four families’ from Barcarena and Breves Counties, Pará State, BR. The investigation showed 83 nDNA-PCR positive subjects, whereas ELISA and IF revealed 31 positive cases. Due to the broad discrepancy, three independent series of experiments were conducted one year apart. They confirmed 76% (83/109) positive nDNA-PCR and 28.4% (31/109) positive ELISA and IF serum samples. The nDNA-PCR also demonstrated T. cruzi infection in 82.6% (19/23) of semen samples. The presence of T. cruzi in semen suggested the infection can be transmitted sexually in humans12, 13. Of interest, the absence of T. cruzi antibody in 63.6% (53/83) of those with positive nDNA-PCR is explained by immune tolerance demonstrated in the chicken early embryo model system14. In addition, a field study comprising 36 members of two families from São Felipe County, Bahia, Brazil, revealed nDNA-PCR positive in 61% (22/36) of cases, whereas ELISA and IF were positive in 22% (8/36) of individuals. Two independent investigations conducted in the family’ studies in two ecosystems at different occasions confirmed the broad discrepancies among the ratios of positive antibody assays and those obtained by the nucleic acid test: the antibody tests were positive in 26.9% (39/145) subjects, whereas the Trypanosoma cruzi nuclear DNA-PCR was positive in 72.4% (105/145) of blood samples tested.
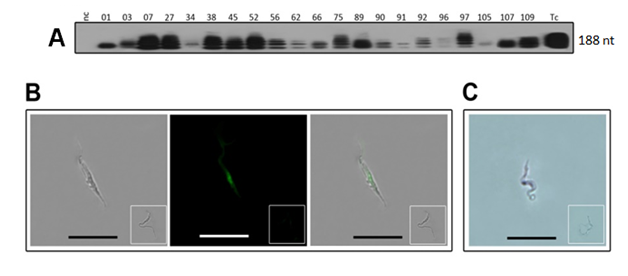
Figure 3 The genotype of Trypanosoma cruzi isolates from acute Chagas disease shown by nucleic acids assay. A) nDNA-PCR 188-nt bands formed after Southern hybridization with the specific radio labeled probe. B) The T. cruzi bright-field image depicted the parasite trypomastigote visible with a primary IgG to the digoxigenin labeled probe after incubation with a secondary fluorescent antibody, and merge of previous images. C) The T. cruzi hybridization with the specific 188-nt digoxigenin-labeled probe, showing dark nucleus stained by an alkaline phosphatase-labeled monoclonal Ab to digoxigenin. Inserts show negative controls. Reprinted with permissions from the Author and the Publisher.12
Chagas disease requires skilled personnel at the clinical center, hospital, and blood bank in order to disclaim donation candidate with basis on epidemiologic history, clinic evaluation, and ELISA serology and nucleic acids test. In view of insurmountable demand, quick and accurate nucleic acids diagnosis is essential for disclaim blood donor candidate. The development of an automated digital platform for throughput nucleic acid diagnostics is essential for blood donor disclaimers. The up-date technology proposal of molecular diagnosis will bring in short term low-cost prevention of Chagas disease.
Not applicable.
The work received financial support grant 482116/2012-9 from the National Research Council-CNPq, Brazil.
The authors declare no conflicts of interest.
Our deepest thanks to the Institute of Biological Sciences of Federal University of Goiás, and to the Faculty of Medicine of the University of Brasília for the technical support.

©2019 Gomes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.