Journal of
eISSN: 2469 - 2786


Mini Review Volume 6 Issue 1
1Key Laboratory of Pathogenic Fungi and Mycotoxins of Fujian Province, Fujian Agriculture and Forestry University, China
2Key Laboratory of Biopesticide and Chemical Biology of Education Ministry, and School of Life Science, Fujian Agriculture and Forestry University, China
3Department of Forestry and Range Management, Arid Agriculture University, Pakistan
4Key Laboratory of Agroecological Processing and Safety Monitoring, Fujian Agriculture and Forestry University, China
5College of Horticulture Ornamental Plants breeding and genetics, Fujian Agriculture and Forestry University, China
Correspondence: Saleem Ahmad, Key Laboratory of Pathogenic Fungi and Mycotoxins of Fujian Province, Fujian Agriculture and Forestry University, China
Received: July 31, 2017 | Published: January 8, 2018
Citation: Ahmad S, Raqeeb A, Ali F, et al. Characterization of novel antibiotic resistance genes in Staphylococcal aureus. J Bacteriol Mycol Open Access. 2018;6(1):8-10. DOI: 10.15406/jbmoa.2018.06.00167
Despite many efforts to control resistance phenomenon, antibiotic resistance in Staphylococcal aureus. The evolution of increasingly antimicrobial resistant in Staphylococcus aureus stems from a multitude factors that involve the widespread and sometimes unsuitable utilize of antimicrobials. The use of Methicillin and Vancomycin were very effective against Staph aureus, but using genetic versatility they have adapted resistance to these antibiotics, and methicillin resistance and vancomycin resistance strains developed. Resistance can be to a quantity of extent contained by a smaller amount and better use of antibiotics, but ultimately novel molecular mechanisms required for treatment and control of antibiotic resistance. The present study will focus on the identification of novel genes and their mechanism in S. aureus strains and focus on some new targets for therapeutic agents against these antibiotic resistance strains.
Keywords: staphylococcal aureus; antibiotic; methicillin; vancomycin; therapeutic
S. aureus is possibly the pathogen of most concern because of its intrinsic virulence, its ability to cause a diverse array of life threatening infections, and its capacity to adapt to different environmental conditions.1 The mortality of S. aureus bacteremia remains just about 20-40% despite the accessibility of effective antimicrobials. S. aureus is now the leading source of nosocomial infections and, as more patients a treated outside the hospital setting, is a rising concern in the community.2,3 Recent reports of S. aureus isolates with intermediate, and whole resistance to vancomycin portend a chemotherapeutic era in which effectual bactericidal antibiotics against this organism may no longer be available.4 The irony of this trend toward progressively more resistant bacteria is that it coincides with a period of increased understanding of the molecular mechanisms of antimicrobial resistance. Unfortunately, while this insight has resulted in the identification of novel drug targets, it has not yet resulted in effective new chemotherapeutic agents.
The introduction of penicillin in the near the beginning the 1940s considerably improved the prognosis of patients with Staphylococcal infection. Though, as near the beginning as 1942, penicillin-resistant staphylococci were recognized, first in hospitals and subsequently in the community.5 The late 1960s, further more 80% of both Community, as well as hospital, acquired staphylococcal isolates were resistant to penicillin. More than 90% of staphylococcal isolates at present generate penicillinase, regardless of the clinical setting.2
Methicillin was the first of the semisynthetic penicillinase resistant penicillins introduced in 1961, Its introduction quickly followed by reports of methicillin-resistant isolates.6 Also, the existence of virulence genes such as the enterotoxins or the Panton-Valentine leukocidin, the mecA gene is the main agent for methicillin resistance and is part of a mobile genetic element found in all MRSA strains,7 demonstrated that mec A is part of a genomic island designated staphylococcal cassette chromosome mec (SCCmec).
The recent upsurge of community acquired MRSA infections reported in patients from dissimilar countries associated with the finding of a unique SCCmec, type IV.8 This element, smaller than the other elements, appears more genetically mobile and does not carry extra antimicrobial resistance genes. It also appears to occur in an additional varied range of MSSA genetic backgrounds, signifying that it has been heterologously transferred additional readily from further staphylococcal species.9 Staphylococcal resistance to vancomycin in a clinical isolate first reported in a strain of Staphylococcus haemolyticus.10 The initial report of vancomycin intermediate-resistant
S. aureus (VISA) In 1997 came from Japan, and supplementary cases afterward reported from other countries. The VISA isolates were every one MRSA and were not clonal. Numerous of the patients had received vancomycin therapy and had MRSA infections.11 Fluoroquinolones was at first introduced for the conduct of Gram-negative bacterial infections in the 1980s.12 Due to their Gram-positive bacterial spectrum, they have as well used to diagnose bacterial infections caused by pneumococci and staphylococci.2
Staphylococcal is mediated by blaZ, resistance to penicillin, the gene encodes β-lactamase. The gene is part of a transposable element located on a large plasmid, often with additional antimicrobial resistance genes. This predominantly extracellular enzyme, synthesized while staphylococci are showing to β-lactam antibiotics, hydrolyzes the β-lactam ring, rendering the β-lactam inactive. The blaZ is under the control of two adjacent regulatory genes, the antirepressor blaR1 and the repressor blaI.11 Current studies have verified that the signaling pathway responsible for β-lactamase synthesis requires sequential cleavage of the regulatory proteins BlaR1 and BlaI (Figure 1).
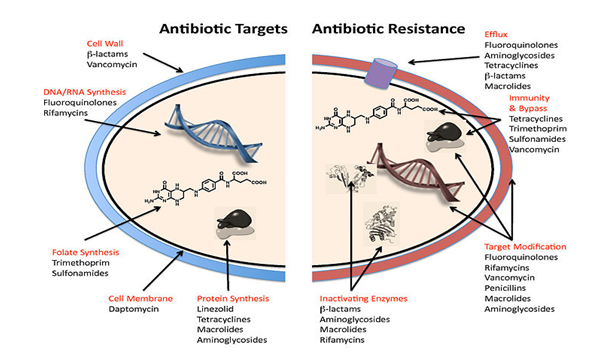
Figure 1 A number of mechanism used by the common antibiotics to deal with bacteria and ways by which bacteria become resistant to them.
The methicillin resistance requires the presence of the chromosomally localized mecA gene.13 The mecA is responsible for the synthesis of penicillin-binding protein 2a (also called PBP2′) a 78-kDa protein. PBPs are membrane bound enzymes that catalyze the trans peptidation reaction that is necessary for cross-linkage of peptide glycan chains.13 There are two forms of S. aureus resistance to vancomycin have at present recognized. One form has identified in the VISA strains, which contain MICs to vancomycin of 8-16µg/ml.9 A pre-VISA phase of resistance, heterogeneously resistant, has furthermore been identified. The hetero-resistant strains continue susceptible to vancomycin but contain resistant subpopulations. It hypothesized that, on disclosure to vancomycin, the VISA isolates selected from the vancomycin-resistant subpopulations. The reduced susceptibility to vancomycin appears to the outcome from changes in peptidoglycan biosynthesis.14 The VISA strains are notable for the extra quantities of synthesized peptidoglycan that result in irregularly shaped, thickened cell walls. The second type of vancomycin resistance has resulted from the possible conjugal transmit of the vanA operon from a vancomycin-resistant E. faecalis reported that the enterococcal plasmid contains vanA as well encodes a sex pheromone, which synthesized by S. aureus, suggesting a potential facilitator of conjugal transfer.14
Quinolone resistance among S. aureus emerged rapidly, additional prominently among the methicillin-resistant strains. As a result, the capability to use fluoroquinolones as antistaphylococcal agents dramatically reduced.15 The reasons for the disparity in rates of quinolone resistance between MSSA and MRSA strains are unsure. One causative factor is likely antibiotic selective pressure, particularly in the hospital setting, resulting in the choice and spread of the extra antibiotic-resistant MRSA strains resistance to quinolones outcome from the stepwise acquisition of chromosomal mutations. The confluence of high bacterial density, the possible pre existence of resistant sub populations, and the from time to time limited quinolone concentrations achieved at sites of staphylococcal infections create an environment that fosters selection of resistant mutants.11
The supply of innovative agents with novel mechanisms of action is limited, however, emphasizes the need for the development of new drug targets.16 Unfortunately, an increasing number of pharmaceutical companies have either eliminated or dramatically reduced their anti-infective units. This outcome partly from economic considerations but also from frustration that target-based biochemical screening has unsuccessful to build up some clinically useful products.
The failure has been recognized, in part to the recognition that target-based strategies do not get into account the intrinsic mechanisms of bacterial resistance (biofilms, multidrug efflux pumps) to contribute in vivo bacterial resistance.17 Despite these developments, some attractive models for recognition of novel drug targets have emerged.
One approach has been to integrate genomic instructions and discover novel resistance genes on possible drug targets with a high-throughput screening followed by chemical modification and efficacy in an animal model. There has been a transformed attention in the characterization of essential components of serious biosynthetic pathways (fatty acid biosynthesis or peptidoglycan assembly) as potential targets.18 Many dissimilar techniques, as well as in vivo expression technology, signature-tagged mutagenesis, and detection of expressed S. aureus antigens, have been used to recognize potential targets that expressed during infection. Examination of the crystal structure of drug targets (modifications of β-lactams that hit the active site of PBP2a) also the synthesis of carbohydrate-modified compounds (glycopeptide analogs among altered carbohydrates) are gradually more used to develop alternative agents.19 Phenol-soluble modules and S. adenosyl homocysteine nucleosidase can serve as anti-staphylococcal targets.20 Modification of S. aureus genes linked with virulence reduces infectivity.21,22 Whether these genes can effectively use as potential targets is uncertain.
The study determines the possible antibiotic targets that can improve the efficacy of pre-existing antibiotic and would propose an idea for the new antibacterial. Some new mechanisms of resistance will discover that can further elaborate S. aureus pathogenesis and will help in other pathogens virulence. We propose new molecular pathways that can reduce the emergence of antibiotic resistance in S. aureus.
None.
The author declares no conflict of interest.

©2018 Ahmad, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.